Abstract
Background
Type 2 diabetes mellitus (T2DM) is often associated with metabolic disorders. Statin drugs are potent inhibitors of cholesterol biosynthesis.
Objective
The aim of this study was to evaluate the effect of atorvastatin on the concentrations of methylglyoxal (MGO), glyoxalase 1 (GLO-1), and aldo–keto reductase family 1 member B10 (AKR1B10) in patients with T2DM and prediabetes.
Methods
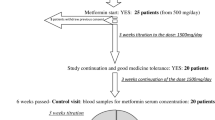
This study was conducted on 80 subjects with and without T2DM and prediabetics divided into 5 groups: patients with T2DM receiving statins (group A, n = 17), patients with T2DM not receiving statins (group B, n = 17), patients with prediabetes receiving statins (group C, n = 12), patients with prediabetes not receiving statins (group D, n = 17), and healthy controls without T2DM (control group, n = 17). Patients with T2DM and prediabetes received atorvastatin 20 mg/day for 3 months. The measurement of MGO and AKR1B10 was performed with a non-competitive sandwich-type enzyme-linked immunosorbent assay (ELISA) at 450 nm. The measurement of GLO-1 was performed by an enzymatic method at 240 nm.
Results
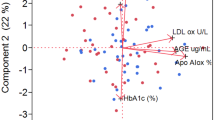
The serum level of MGO was significantly higher in patients with T2DM and prediabetes than that of healthy controls (p = 0.001). In patients with T2DM, statins decreased the serum level of MGO, but in patients with prediabetes, statins increased the serum level of MGO (p = 0.001). The level of GLO-1 activity was significantly higher in healthy controls than that of patients with T2DM and prediabetes (p = 0.001). Furthermore, the level of GLO-1 activity was significantly higher in patients with T2DM and prediabetes receiving statins than that of patients with T2DM and prediabetes not receiving statins (p = 0.002). The serum level of AKR1B10 was significantly higher in groups C and D than that of the other groups (p = 0.001).
Conclusion
Atorvastatin can improve the level of GLO-1 activity and thereby prevent diabetic complications.





Similar content being viewed by others
Data availability
No additional data are available.
References
Centers for Disease Control and Prevention (CDC), et al. Awareness of prediabetes--United States, 2005-2010. Morbidity and Mortality Weekly Report (MMWR). 2013;62(11):209.
Lily M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009;55(4):363–9.
Wang N, Cheng J, Ning Z, Chen Y, Han B, Li Q, et al. Type 2 diabetes and adiposity induce different lipid profile disorders: a mendelian randomization analysis. J Clin Endocrinol Metab. 2018;103(5):2016–25.
Qujeq D, Mahrooz A, Alizadeh A, Masoumi P, Annemohammadzadeh S, Boorank R. Genotype and phenotype of salt-stimulated paraoxonase 1 (PON1) is associated with atherogenic indices in type 2 diabetes. J Diabetes Metab Disord. 2018;17(1):1–10.
Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96(5):24–33.
Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton JVP, Ridker PM. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA. 2004;291(23):2821–7.
Glorioso N, Troffa C, Filigheddu F, Dettori F, Soro A, Parpaglia PP, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34(6):1281–6.
Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203(2):325–30.
White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42(9):963–70.
Agouridis AP, Kostapanos MS, Elisaf MS. Statins and their increased risk of inducing diabetes. Expert Opin Drug Saf. 2015;14(12):1835–44.
Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal. 2014;20(8):1251–67.
Aiman U, Najmi A, Khan RA. Statin induced diabetes and its clinical implications. J Pharmacol Pharmacother. 2014;5(3):181.
Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame. Circ Res. 2006;98(6):730–42.
Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal. 2014;20(8):1251–67.
Kwok JM, Ma CCH, Ma S. Recent development in the effects of statins on cardiovascular disease through Rac1 and NADPH oxidase. Vascul Pharmacol. 2013;58(1):21–30.
Rashid M, Tawara S, Fukumoto Y, Seto M, Yano K, Shimokawa H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ J. 2009;73(2):361–70.
Babelova A, Jansen F, Sander K, Löhn M, Schäfer L, Fork C, et al. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS ONE. 2013;8(11): e80328.
Tanaka SI, Fukumoto Y, Nochioka K, Minami T, Kudo S, Shiba N, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation significance. Arterioscler Thromb Vasc Biol. 2013;33(7):1591–600.
Margaritis M, Sanna F, Antoniades C. Statins and oxidative stress in the cardiovascular system. Curr Pharm Des. 2017;23(46):7040–7.
Den Hartog G, Chattopadhyay R, Ablack A, Hall EH, Butcher LD, Bhattacharyya A, et al. Regulation of Rac1 and reactive oxygen species production in response to infection of gastrointestinal epithelia. PLoS Pathog. 2016;12(1): e1005382.
Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58(10):2386–95.
** S, Ray RM, Johnson LR. TNF-α/cycloheximide-induced apoptosis in intestinal epithelial cells requires Rac1-regulated reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):928–37.
Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S, et al. Induction of endothelial nitric oxide synthase, Sirt1, and catalase by statins inhibits endothelial senescence through the AKT pathway. Arterioscler Thromb Vasc Biol. 2010;30(11):2205–11.
Maessen DE, Hanssen NM, Scheijen JL, van der Kallen CJ, van greevenbroek MM, Stehouwer CD, Schalkwijk CG. Post–glucose load plasma α-dicarbonyl concentrations are increased in individuals with impaired glucose metabolism and type 2 diabetes: the CODAM study. Diabetes Care. 2015;38(5):913–20.
Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(10):4867–74.
Maessen DE, Hanssen NM, Lips MA, Scheijen JL, van Dijk KW, Pijl H, et al. Energy restriction and Roux-en-Y gastric bypass reduce postprandial α-dicarbonyl stress in obese women with type 2 diabetes. Diabetologia. 2016;59(9):2013–7.
Brouwers O, Niessen PM, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57(1):224–35.
Chen HJC, Chen YC, Hsiao CF, Chen PF. Mass spectrometric analysis of glyoxal and methylglyoxal-induced modifications in human hemoglobin from poorly controlled type 2 diabetes mellitus patients. Chem Res Toxicol. 2015;28(12):2377–89.
Beisswenger PJ. Methylglyoxal in diabetes: link to treatment, glycaemic control and biomarkers of complications. Biochem Soc Trans. 2014;42(2):450–6.
Dornadula S, Elango B, Balashanmugam P, Palanisamy R, Kunka MR. Pathophysiological insights of methylglyoxal induced type-2 diabetes. Chem Res Toxicol. 2015;28(9):1666–74.
Tang W, Martin KA, Hwa J. Aldose reductase, oxidative stress and diabetic mellitus. Front Pharmacol. 2012;3:87.
Van Boekel MAJS. Formation of flavour compounds in the maillard reaction. Biotechnol Adv. 2006;24(2):230–3.
Hayashi CM, Nagai R, Miyazaki K, Hayase F, Araki T, Ono T, Horiuchi S. Conversion of amadori products of the maillard reaction to N [straight epsilon]-(carboxymethyl) lysine by short-term heating: possible detection of artifacts by immunohistochemistry. Lab Invest. 2002;82(6):795.
Turk Z. Glycation and complications of diabetes. Diabetologia Croat. 2001;30(2):49–54.
Hayase F. Recent development of 3-deoxyosone related maillard reaction products. Food Sci Technol Res. 2007;6(2):79–86.
Semchyshyn HM. Fructation in vivo: detrimental and protective effects of fructose. Biomed Res Int. 2013;2013: 343914.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(1):109–16.
Moriyama T, Kawada N, Nagatoya K, Takeji M, Horio M, Ando A, et al. Fluvastatin suppresses oxidative stress and fibrosis in the interstitium of mouse kidneys with unilateral ureteral obstruction. Kidney Int. 2001;59(6):2095–103.
Lange JN, Wood KD, Knight J, Assimos DG, Holmes RP. Glyoxal formation and its role in endogenous oxalate synthesis. Adv Urol. 2012;2012: 819202.
Masania J, Malczewska-Malec M, Razny U, Goralska J, Zdzienicka A, Kiec-Wilk B, et al. Dicarbonyl stress in clinical obesity. Glycoconj J. 2016;33(4):581–9.
Bair WB III, Cabello CM, Uchida K, Bause AS, Wondrak GT. GLO1 overexpression in human malignant melanoma. Melanoma Res. 2010;20(2):85.
Hirakawa Y, Inagi R. Glycative stress and its defense machinery glyoxalase 1 in renal pathogenesis. Int J Mol Sci. 2017;18(1):174.
Rabbani N, Thornalley PJ. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun. 2015;458(2):221–6.
Weber J, Kayser A, Rinas U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology. 2005;151(3):707–16.
Huang SP, Palla S, Ruzycki P, Varma RA, Harter T, Reddy GB, Petrash JM. Aldo-keto reductases in the eye. J Ophthalmol. 2010;2010: 521204.
Muthenna P, Suryanarayana P, Gunda SK, Petrash JM, Reddy GB. Inhibition of aldose reductase by dietary antioxidant curcumin: mechanism of inhibition, specificity and significance. FEBS Lett. 2009;583(22):3637–42.
Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40(4):553–624.
Shaw N, Yang B, Millward A, Demaine A, Hodgkinson A. AKR1B10 is induced by hyperglycaemia and lipopolysaccharide in patients with diabetic nephropathy. Cell Stress Chaperones. 2014;19(2):281–7.
Nikiforova VJ, Giesbertz P, Wiemer J, Bethan B, Looser R, Liebenberg V, et al. Glyoxylate, a new marker metabolite of type 2 diabetes. J Diabetes Res. 2014;2014:685204.
Kain V, Kapadia B, Misra P, Saxena U. Simvastatin may induce insulin resistance through a novel fatty acid mediated cholesterol independent mechanism. Sci Rep. 2015;5:13823.
Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89(3):604–61.
Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37(3):635–46.
Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet β-cells. Br J Pharmacol. 1999;126(5):1205–13.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–42.
Agouridis AP, Kostapanos MS, Elisaf MS. Statins and their increased risk of inducing diabetes. Expert Opin Drug Saf. 2015;14(12):1835–44.
Tsuchiya M, Hosaka M, Moriguchi T, Zhang S, Suda M, Yokota-Hashimoto H, et al. Cholesterol biosynthesis pathway intermediates and inhibitors regulate glucose-stimulated insulin secretion and secretory granule formation in pancreatic β-cells. Endocrinology. 2010;151(10):4705–16.
Giacco F, Du X, D’agati VD, Milne R, Sui G, Geoffrion M, Brownlee M. Knockdown of Glo1 mimics diabetic nephropathy in non-diabetic mice. Diabetes. 2014;63(1):291–9.
Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286(2):1374–80.
Ruf TF, Quintes S, Sternik P, Gottmann U. Atorvastatin reduces the expression of aldo-keto reductases in HUVEC and PTEC. A new approach to influence the polyol pathway. Clin Investig Med. 2009;32(3):219–28.
Acknowledgements
This work was financially supported by Hormozgan University of Medical Sciences. We are grateful to Dr Mir and Dr Karimian for their technical assistance in biochemical assays.
Author information
Authors and Affiliations
Contributions
DQ designed the experiments. AN performed the experiments. KH analyzed the data, and SM contributed to the writing and revising of the manuscript.
Corresponding authors
Ethics declarations
Institutional review board statement
The study was approved by the Ethics Committee of Hormozgan University of Medical Sciences.
Arrive guidelines statement
The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Conflict of interest
The authors declare no conflict of interests.
Ethical clearance and consent of participation
This study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (HUMS.REC.1395.127). Written consent was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andevari, A.N., Moein, S., Qujeq, D. et al. The effect of atorvastatin on the concentrations of methylglyoxal, glyoxalase 1, and aldo–keto reductase family 1 member B10 in patients with type 2 diabetes mellitus and prediabetes. Int J Diabetes Dev Ctries 44, 400–408 (2024). https://doi.org/10.1007/s13410-023-01249-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01249-6