Abstract
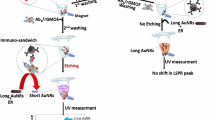
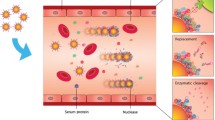
Conjugation and characterization of poly-ethylene–glycol (PEG)-functionalized gold nanourchin (GNU) with breast cancer biomarker HER-II monoclonal antibody (mAb) (i.e., anti-HER-II) for selective targeting are described. After the functionalization of GNU with PEG, the surface plasmon resonance (SPR) peak was red-shifted, indicating the increase in the hydrodynamic size of the GNU. The Fourier-transform near-infrared spectroscopy (FT-NIR) second derivative result of GNU-PEG provided overtone and combination bands of fundamental vibrational modes of protein molecular structures between 4000 and 7500 cm−1. This mainly included C–H combination and CH2 bonds, O–H first stretch overtones, the C–H first stretch overtone, and the CH2 combination first overtone. The UV–Vis absorbance showed a strong absorption of light at 227 and 275 nm corresponding to tyrosine peaks. The fluorescence emission peak at 315 nm corresponds to Stokes shift when excited by 280 nm corresponding to tyrosine in the mAb, and the peak at 497 nm likely corresponds to alanine. After conjugation of GNU-PEG with mAb, the FT-NIR indicated the bands corresponding to NH2 combination and amino acids, first overtone symmetric and antisymmetric OH stretching, C–H combination, and the second overtones and combination modes. Surface-enhanced Raman scattering (SERS) provided useful information on the molecular structure and composition of the sample within 300–3500 cm−1. The intensity behavior of SERS signals exhibited a statistical nature due to Brownian fluctuating movement. In addition, the intensity and number of SERS lines varied with the laser power. The dominant peaks were corresponding to histidine, tyrosine, tryptophan, phenylalanine, and C–H, N–H, C–N, and O–H bonds.








Similar content being viewed by others
References
Bao G, Mitragotri S, Sheng T (2013) Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng 15:253–282. https://doi.org/10.1146/annurev-bioeng-071812-152409
Sinha R, Gloria J, Nie S, Shin DM (2006) Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther 5:1909–1917. https://doi.org/10.1158/1535-7163.MCT-06-0141
Khosroshahi ME, Ghazanfari L, Hassannejad Z, Lenhert S (2015) In-vitro application of doxorubicin loaded magnetoplasmonic thermosensitive liposomes for laser hyperthermia and chemotherapy of breast cancer. J Nanomed Nanotechnol 6:1–9. https://doi.org/10.4172/2157-7439.1000298
Urban C, Urban AS, Charron H, Joshi A (2013) Externally modulated theranostic nanoparticles. Transl Cancer Res 2:292–308. https://doi.org/10.3978/j.issn.2218-676X.2013.08.05
Yang J, Zhang X, Liu C, Wang Z, Deng L, Feng C, Tao W, Xu X, Cui W (2021) Biologically modified nanoparticles as theranostic bionanomaterials. Prog Mater Sci 118:100768. https://doi.org/10.1016/j.pmatsci.2020.100768
Loeb KR, Loeb LA (2000) Significance of multiple mutations in cancer. Carcinogenesis 21:379–385. https://doi.org/10.1093/carcin/21.3.379
Siegel RL, Jamel A, Wander RC et al (2018) An assessment of progress in cancer control. CA Can J Clin 68:329–339. https://doi.org/10.3322/caac.21460
Fan L, Strasser-Weippl K, Li J, St Louis J, Finkelstein D, Yu K et al (2014) Breast cancer in China. Lancet Oncol 15:e279–e289. https://doi.org/10.1016/S1470-2045(13)70567-9
Momenimovahed Z, Salehiniya H (2019) Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 11:151–164. https://doi.org/10.2147/BCTT.S176070
Zhang P, **ao J, Ruan Y, Zhang Z, Zhang X (2020) Monitoring value of serum HER2 as a predictive biomarker in patients with metastatic breast cancer. Cancer Manag Res 12:4667–4675. https://doi.org/10.2147/CMAR.S254897
Tanaka T, Decuzzi P, Cristofanilli M et al (2008) Nanotechnology for breast cancer therapy. Biomed Microdevices 11:49–63. https://doi.org/10.1007/s10544-008-9209-0
Handy B (2009) The clinical utility of tumor markers. Lab Med 40:99–103. https://doi.org/10.1309/LMTRKSKYW4GI6SBJ
Ludwig JA, Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5:845–856. https://doi.org/10.1038/nrc1739
Sano K, Mitsunaga M, Nakajima T, Choyke PL, Kobayashi H (2012) In vivo breast cancer characterization imaging using two monoclonal antibodies activatably labeled with near infrared fluorophores. Breast Can Res 14:R61. https://doi.org/10.1186/bcr3167
Pitsillides CM, Joe EK, Wei X, Anderson RR, Lin CP (2003) Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys J 84:4023–4032. https://doi.org/10.1016/S0006-3495(03)75128-5
Jiang S, Win KY, Liu S, Teng CP, Zheng Y, Han M (2013) Surface-functionalized nanoparticles for biosensing and imaging-guided therapeutics. Nanoscale 5:3127–3148. https://doi.org/10.1039/c3nr34005h
Willets KA, Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58:267–297. https://doi.org/10.1146/annurev.physchem.58.032806.104607
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA (2006) Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B 110:7238–7248. https://doi.org/10.1021/jp057170o
Noguez C (2007) Surface plasmons on metal nanoparticles: the influence of shape and physical environment. J Phys Chem 111:3806–3819. https://doi.org/10.1021/jp066539m
Baffou G, Quidant R (2013) Thermo-plasmonics: using metallic nanostructures as nano-sources of heat. Laser Photonics Rev 7:171–187. https://doi.org/10.1002/lpor.201200003
Hasannejad Z, Khosroshahi ME (2013) Synthesis and evaluation of time dependent optical properties of plasmonic-magnetic nanoparticles. Opt Mater 35:644–651. https://doi.org/10.1016/j.optmat.2012.10.019
Khlebtsov B, Zharov V, Melnikov A, Tuchin V, Khlebtsov N (2006) Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology 17:5167–5179. https://doi.org/10.1088/0957-4484/17/20/022
Hung X, Jain PK, El-Sayed IH, El-Sayed MA (2007) Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2:681–693. https://doi.org/10.2217/17435889.2.5.681
Rodríguez-Oliveros R, Sánchez-Gill JA (2012) Gold nanostars as thermoplasmonic nanoparticles for optical heating. Opt Express 20:621–626. https://doi.org/10.1364/OE.20.000621
Liu Y, Kersey F, Register J, Parrott M (2015) Plasmonic gold nanostars for multi-modality sensing and diagnostics. Sensors 15:3706–3720. https://doi.org/10.3390/s150203706
Zhou W, Gao X, Liu D, Chen X (2019) God nanoparticles in-vitro diagnostics 115:10575–10636. https://doi.org/10.1021/acs.chemrev.5b00100
Ishigaki M, Ozaki Y (2020) Vibrational spectroscopy in protein research. Academic Press, pp 143–176
Iosin M, Toderas F, Baldeck PL, Astilean S (2009) Study of protein-gold nanoparticle conjugates by fluorescence and surface-enhanced Raman scattering. J Mol Struct 924:196–200. https://doi.org/10.1016/j.molstruc.2009.02.004
Mandal G, Bardhan M, Ganguly T (2010) Interaction of bovine serum albumin and albumin-gold nanoconjugates with L-aspartic acid. A spectroscopic approach. Colloids Surf B 81:178–184. https://doi.org/10.1016/j.colsurfb.2010.07.002
Stuart BH (2004) Infrared spectroscopy: fundamentals and applications. Wiley, Chichester
Eldin AB (2011) Near Infra Red spectroscopy. In: Akyar I (ed) Wide spectra of quality control. Intech Open, London, pp 237–247
Giacomelli CE, Bremer MGEG, Norde W (1999) ATR-FTIR study of IgG adsorbed on different silica surfaces. J Colloid Interface Sci 220:13–23. https://doi.org/10.1006/jcis.1999.6479
Van Stokkum IHM, Lindsell H, Hadden JM, Haris PI, Chapman D, Bloemendal M (1995) Temperature-induced changes in protein structures studied by Fourier transform infrared spectroscopy and global analysis. Biochemistry 34:10508–10518. https://doi.org/10.1021/bi00033a024
Izutsu K, Fujimaki Y, Kuwabara A, Hiyama Y, Yomota C, Aoyagi N (2006) Near-infrared analysis of protein secondary structure in aqueous solutions and freeze-dried solids. J Pharm Sci 95:781–789. https://doi.org/10.1002/jps.20580
Tipson RS (1968) Infrared spectroscopy of carbohydrates: a review of the literature. NBS Publications, Washington, pp 1–12
Bjerneld EJ, Földes-Papp Z, Käll M, Rigler R (2002) Single-molecule surface-enhanced Raman and fluorescence correlation spectroscopy of horseradish peroxidase. J Phys Chem B 106:1213–1218. https://doi.org/10.1021/jp012268y
Johannessen C, Abdali S, White PC (2007) Resonance Raman optical activity and surface enhanced resonance Raman optical activity analysis of cytochrome c. J Phys Chem A 111:7771–7776. https://doi.org/10.1021/jp0705267
Winuprasith T, Suphantharika M, McClements DJ, He L (2014) Spectroscopic studies of conformational changes of β-lactoglobulin adsorbed on gold nanoparticle surfaces. J Colloid Interface Sci 416:184–189. https://doi.org/10.1016/j.jcis.2013.11.006
Vargas-Obieta E, Martínez-Espinosa JC, Martínez-Zerega BE, Jave-Suárez LF, Aguilar-Lemarroy A, González-Solís JL (2016) Breast cancer detection based on serum sample surface enhanced Raman spectroscopy. Lasers Med Sci 31:1317–1324. https://doi.org/10.1007/s10103-016-1976-x
Ortiz-Dosal A, Loredo-García E, Álvarez-Contreras AG, Núñez-Leyva JM, Ortiz-Dosal LC, Kolosovas-Machuca ES, Young S (2021) Determination of the Immunoglobulin G spectrum by surface-enhanced Raman spectroscopy using quasispherical gold nanoparticles. J Nanomater 21. https://doi.org/10.1155/2021/8874193
Szekeres GP, Kneipp J (2019) SERS probing of proteins in gold nanoparticle agglomerates. Front Chem 7:30. https://doi.org/10.3389/fchem.2019.00030
Lambert JB, Shurvell HF (1987) Introduction to organic spectroscopy. Macmillan, New York, pp 174–177, 468
Macdonald IDG, Smith WE (1996) Orientation of cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir 12:706–713. https://doi.org/10.1021/la950256w
Funding
The authors would like to thank MIS Electronics Inc., Department of R&D for supporting the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khosroshahi, M.E., Patel, Y. & Chabok, R. Characterization of breast cancer antibody (anti-HER-II) conjugated on PEGylated gold nanourchin for active targeting. Gold Bull 55, 149–159 (2022). https://doi.org/10.1007/s13404-022-00316-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-022-00316-w