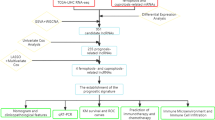
Abstract
Objective
Gastric cancer (GC) is one of the most malignant tumors worldwide. Thus, it is necessary to explore the underlying mechanisms of GC progression and develop novel therapeutic regimens. Long non-coding RNAs (lncRNAs) have been demonstrated to be abnormally expressed and regulate the malignant behaviors of cancer cells. Our previous research demonstrated that lncRNA colon cancer-associated transcript 2 (CCAT2) has potential value for GC diagnosis and discrimination. However, the functional mechanisms of lncRNA CCAT2 in GC development remain to be explored.
Methods
GC and normal adjacent tissues were collected to detect the expression of lncRNA CCAT2, ESRP1 and CD44 in clinical specimens and their clinical significance for GC patients. Cell counting kit-8, wound healing and transwell assays were conducted to investigate the malignant behaviors in vitro. The generation of nude mouse xenografts by subcutaneous, intraperitoneal and tail vein injection was performed to examine GC growth and metastasis in vivo. Co-immunoprecipitation, RNA-binding protein pull-down assay and fluorescence in situ hybridization were performed to reveal the binding relationships between ESRP1 and CD44.
Results
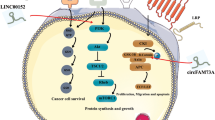
In the present study, lncRNA CCAT2 was overexpressed in GC tissues compared to adjacent normal tissues and correlated with short survival time of patients. lncRNA CCAT2 promoted the proliferation, migration and invasion of GC cells. Its overexpression modulates alternative splicing of Cluster of differentiation 44 (CD44) variants and facilitates the conversion from the standard form to variable CD44 isoform 6 (CD44v6). Mechanistically, lncRNA CCAT2 upregulated CD44v6 expression by binding to epithelial splicing regulatory protein 1 (ESRP1), which subsequently mediates CD44 alternative splicing. The oncogenic role of the lncRNA CCAT2/ESRP1/CD44 axis in the promotion of malignant behaviors was verified by both in vivo and in vitro experiments.
Conclusions
Our findings identified a novel mechanism by which lncRNA CCAT2, as a type of protein-binding RNA, regulates alternative splicing of CD44 and promotes GC progression. This axis may become an effective target for clinical diagnosis and treatment.






Similar content being viewed by others
Data availability
Data are available from corresponding authors from reasonable request.
References
H. Sung, J. Ferlay, R. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 71(3), 209–249 (2021)
M. Sun, H. Ding, Z. Zhu, S. Wang, X. Gu, L. **a et al., Identifying optimal surgical intervention-based chemotherapy for gastric cancer patients with liver metastases. Front. Oncol. 11, 675870 (2021)
F. Shi, T. Li, Z. Liu, K. Qu, C. Shi, Y. Li et al., FOXO1: another avenue for treating digestive malignancy? Semin. Cancer Biol. 50, 124–131 (2018)
X. Zhang, S. Wang, H. Wang, J. Cao, X. Huang, Z. Chen et al., Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. 18(1), 20 (2019)
B. Cao, G. Liu, W. Zhang, Y. Shi, B. Wei, Role of circular RNAs and long noncoding RNAs in the clinical translation of gastric cancer (review). Int. J. Mol. Med. 47(1), 77–91 (2021)
Z. **ang, Y. Liqing, Y. Qingqing, H. Qiang, C. Hongbo, Retard or exacerbate: role of long non-coding RNA growth arrest-specific 5 in the fibrosis. Cytokine Growth Factor Rev. 67, 89–104 (2022)
N. Ebrahimi, S. Parkhideh, S. Samizade, A.N. Esfahani, S. Samsami, E. Yazdani et al., Crosstalk between lncRNAs in the apoptotic pathway and therapeutic targets in cancer. Cytokine Growth Factor Rev. 65, 61–74 (2022)
J. Yang, Z. Li, L. Wang, X. Yun, Y. Zeng, J.P.L. Ng et al., The role of non-coding RNAs (miRNA and lncRNA) in the clinical management of rheumatoid arthritis. Pharmacol. Res. 186, 106549 (2022)
M. Hashemi, M.S. Moosavi, H.M. Abed, M. Dehghani, M. Aalipour, E.A. Heydari et al., Long non-coding RNA (lncRNA) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol. Res. 184, 106418 (2022)
Z. Bian, J. Zhang, M. Li, Y. Feng, X. Wang, J. Zhang et al., LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin. Cancer Res. 24(19), 4808–4819 (2018)
W. Ni, S. Yao, Y. Zhou, Y. Liu, P. Huang, A. Zhou et al., Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol. Cancer 18(1), 143 (2019)
L. Zhang, W. Kang, X. Lu, S. Ma, L. Dong, B.J.C.c. Zou, LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. 17(15), 1886–1900 (2018)
C. Wang, Y. Li, S. Yan, H. Wang, X. Shao, M. **ao et al., Interactome analysis reveals that lncRNA HULC promotes aerobic glycolysis through LDHA and PKM2. 11(1), 3162 (2020)
M.P. Dragomir, S. Kopetz, J.A. Ajani, G.A. Calin, Non-coding RNAs in GI cancers: from cancer hallmarks to clinical utility. Gut 69(4), 748–763 (2020)
K. Zhang, H. Shi, H. **, X. Wu, J. Cui, Y. Gao et al., Genome-wide lncRNA microarray profiling identifies Novel circulating lncRNAs for detection of gastric cancer. Theranostics 7(1), 213–227 (2017)
S. Lin, H. Wang, W. Yang, A. Wang, C. Geng, Silencing of long non-coding RNA colon cancer-associated transcript 2 inhibits the growth and metastasis of gastric cancer through blocking mTOR signaling. Onco. Targets. Ther. 13, 337–349 (2020)
Y.J. Wang, J.Z. Liu, P. Lv, Y. Dang, J.Y. Gao, Y. Wang, Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am. J. Cancer Res. 6(11), 2651–2660 (2016)
Z.Y. Yu, Z. Wang, K.Y. Lee, P. Yuan, J. Ding, Effect of silencing colon cancer-associated transcript 2 on the proliferation, apoptosis and autophagy of gastric cancer BGC-823 cells. Oncol. Lett. 15(3), 3127–3132 (2018)
R.S. Redis, L.E. Vela, W. Lu, J. Ferreira de Oliveira, C. Ivan, C. Rodriguez-Aguayo et al., Allele-specific reprogramming of cancer metabolism by the long non-coding RNA CCAT2. Mol. Cell 61(4), 520–534 (2016)
H. Xu, M. Niu, X. Yuan, K. Wu, A.J.E.h. Liu, oncology, CD44 as a tumor biomarker and therapeutic target. 9(1), 36 (2020)
Y. Wang, L. Li, Y. Wu, S. Zhang, Q. Ju, Y. Yang et al., CD44 deficiency represses neuroinflammation and rescues dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 177, 106133 (2022)
A. Yim, C. Smith, A.M. Brown, Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-, and neuronal cell ligand-receptor interactions to sense and regulate acute and chronic neuroinflammation. Immunol. Rev. 311(1), 224–233 (2022)
H. Xu, Y. Tian, X. Yuan, H. Wu, Q. Liu, R. Pestell et al., The role of CD44 in epithelial-mesenchymal transition and cancer development. 8, 3783–3792 (2015)
D. Blake, K.W. Lynch, The three as: alternative splicing, alternative polyadenylation and their impact on apoptosis in immune function. Immunol. Rev. 304(1), 30–50 (2021)
I. Moreira, F. Pinto, C. Gomes, D. Campos, C.J.C. Reis, OImpact of truncated -glycans in gastric-Cancer-Associated CD44v9 detection. 9(2) (2020)
Y Zavros, Initiation and maintenance of gastric cancer: a focus on CD44 variant Isoforms and cancer stem cells. Cell Mol. Gastroenterol. Hepatol. 4(1), 55–63 (2017)
B. Jang, Y. Li, D. Graham, P.J.G. Cen, liver, the role of CD44 in the pathogenesis, diagnosis, and therapy of gastric. Cancer 5(4), 397–405 (2011)
B. Preca, K. Bajdak, K. Mock, V. Sundararajan, J. Pfannstiel, J. Maurer et al., A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. 137(11), 2566–2577 (2015)
Y. Gao, A. Cai, H. **, J. Li, W. Xu, Y. Zhang et al., Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-β/catenin signaling pathwa. 8(1), 98 (2017)
C.C. Warzecha, T.K. Sato, B. Nabet, J.B. Hogenesch, R.P. Carstens, ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol. Cell 33(5), 591–601 (2009)
X. Liu, R. Taftaf, M. Kawaguchi, Y.F. Chang, W. Chen, D. Entenberg et al., Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 9(1), 96–113 (2019)
F. Tomizawa, M.K. Jang, T. Mashima, H. Seimiya, c-KIT regulates stability of cancer stemness in CD44-positive colorectal cancer cells. Biochem. Biophys. Res. Commun. 527(4), 1014–1020 (2020)
J. Hu, G. Li, P. Zhang, X. Zhuang, G. Hu, A CD44v(+) subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell. Death Dis. 8(3), e2679 (2017)
T. Ishimoto, O. Nagano, T. Yae, M. Tamada, T. Motohara, H. Oshima et al., CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19(3), 387–400 (2011)
C.C. Tseng, R. Stanciauskas, P. Zhang, D. Woo, K. Wu, K. Kelly et al., GRP78 regulates CD44v membrane homeostasis and cell spreading in tamoxifen-resistant breast cancer. Life Sci. Alliance 2(4) (2019)
X. Zhang, S. Yu, X. Li, X. Wen, S. Liu, R. Zu et al., Research progress on the interaction between oxidative stress and platelets: another avenue for cancer? Pharmacol. Res. 191, 106777 (2023)
F. Zhang, K. Wang, P. Du, W. Yang, Y. He, T. Li et al., Risk of stroke in cancer survivors: a meta-analysis of population-based cohort studies. Neurology 96(4), e513–e526 (2021)
X. Zhao, Z. **ao, B. Li, H. Li, B. Yang, T. Li et al., miRNA-21 may serve as a promising noninvasive marker of glioma with a high diagnostic performance: a pooled analysis of 997 patients. Ther. Adv. Med. Oncol. 13, 1758835920987650 (2021)
J.A. Siddiqui, R. Pothuraju, P. Khan, G. Sharma, S. Muniyan, P. Seshacharyulu et al., Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor Rev. 64, 71–83 (2022)
B. Hu, W. Luo, R.T. Hu, Y. Zhou, S.Y. Qin, H.X. Jiang, Meta-analysis of prognostic and clinical significance of CD44v6 in esophageal cancer. Medicine (Baltimore) 94(31), 1238 (2015)
C. Nicolazzo, F. Loreni, S. Caponnetto, V. Magri, A.R. Vestri, R. Zamarchi et al., Baseline CD44v6-positive circulating tumor cells to predict first-line treatment failure in patients with metastatic colorectal cancer. Oncotarget 11(45), 4115–4122 (2020)
Q.X. Wang, L. Lv, D.R. Ye, Y.H. Sun, X.X. Pan, A. Bhandari et al., Downregulation of CD44v6 enhances chemosensitivity by promoting apoptosis and inhibiting autophagy in colorectal cancer HT29 cells. Ann. Clin. Lab. Sci 49(4), 481–487 (2019)
L. Ma, L. Dong, P. Chang, CD44v6 engages in colorectal cancer progression. Cell Death Dis. 10(1), 30 (2019)
B.W. Eom, J. Joo, B. Park, M.J. Jo, S.H. Choi, S.J. Cho et al., Nomogram incorporating CD44v6 and clinicopathological factors to predict lymph node metastasis for early gastric cancer. PLoS One 11(8), 0159424 (2016)
S. Lobo, C. Pereira, C. Oliveira, G.M. Almeida, Skip** Exon-v6 from CD44v6-Containing Isoforms Influences Chemotherapy Response and Self-Renewal Capacity of Gastric Cancer cells. Cancers (Basel) 12(9) (2020)
C. Pereira, D. Ferreira, N. Mendes, P.L. Granja, G.M. Almeida, C. Oliveira, Expression of CD44v6-Containing Isoforms Influences Cisplatin response in gastric Cancer cells. Cancers (Basel) 12(4) (2020)
F. Zhang, H. Wang, J. Yu, X. Yao, S. Yang, W. Li et al., LncRNA CRNDE attenuates chemoresistance in gastric cancer via SRSF6-regulated alternative splicing of PICALM. Mol. Cancer 20(1), 6 (2021)
Z. Wang, X. Yang, C. Liu, X. Li, B. Zhang, B. Wang et al., Acetylation of PHF5A modulates stress responses and colorectal carcinogenesis through alternative splicing-mediated upregulation of KDM3A. Mol. Cell 74(6), 1250-1263e1256 (2019)
Y.D. Kim, H.S. Kim, J. Lee, J.K. Choi, E. Han, J.E. Jeong et al., ESRP1-Induced CD44 v3 is important for controlling pluripotency in human pluripotent stem cells. Stem Cells 36(10), 1525–1534 (2018)
C.M. Weng, Q. Li, K.J. Chen, C.X. Xu, M.S. Deng, T. Li et al., Bleomycin induces epithelial-to-mesenchymal transition via bFGF/PI3K/ESRP1 signaling in pulmonary fibrosis. Biosci. Rep. 40(1) (2020)
G. Deng, X. Zhou, L. Chen, Y. Yao, J. Li, Y. Zhang et al., High expression of ESRP1 regulated by circ-0005585 promotes cell colonization in ovarian cancer. Cancer Cell Int. 20, 174 (2020)
Z.H. Chen, Y.J. **g, J.B. Yu, Z.S. **, Z. Li, T.T. He et al., ESRP1 induces cervical Cancer Cell G1-Phase arrest Via regulating cyclin A2 mRNA Stability, Int. J. Mol. Sci. 20(15) (2019)
L. Chen, Y. Yao, L. Sun, J. Zhou, M. Miao, S. Luo et al., Snail driving alternative splicing of CD44 by ESRP1 enhances invasion and migration in epithelial ovarian cancer. Cell. Physiol. Biochem. 43(6), 2489–2504 (2017)
S.L. Sun, Y.G. Shu, M.Y. Tao, LncRNA CCAT2 promotes angiogenesis in glioma through activation of VEGFA signalling by sponging miR-424. Mol. Cell. Biochem. 468(1–2), 69–82 (2020)
L. Yan, X. Wu, X. Yin, F. Du, Y. Liu, X. Ding, LncRNA CCAT2 promoted osteosarcoma cell proliferation and invasion. J. Cell. Mol. Med 22(5), 2592–2599 (2018)
M. Wang, L. Wang, X. He, J. Zhang, Z. Zhu, M. Zhang et al., lncRNA CCAT2 promotes radiotherapy resistance for human esophageal carcinoma cells via the miR–145/p70S6K1 and p53 pathway. Int. J. Oncol. 56(1), 327–336 (2020)
G. Wu, J. Ke, J. Wang, X. Zhu, S. **ong, LncRNA CCAT2 promotes the proliferation and invasion of renal cell cancer by sponging miR-320a. Panminerva Med. (2020)
B. Chen, M.P. Dragomir, L. Fabris, R. Bayraktar, E. Knutsen, X. Liu et al., The long noncoding RNA CCAT2 induces chromosomal instability through BOP1-AURKB signaling. Gastroenterology. 159(6), 2146–2162e2133 (2020)
S. Shaid, C.H. Brandts, H. Serve, I. Dikic, Ubiquitination and selective autophagy. Cell Death Differ. 20(1), 21–30 (2013)
W. Lu, F. Cao, L. Feng, G. Song, Y. Chang, Y. Chu et al., LncRNA Snhg6 regulates the differentiation of MDSCs by regulating the ubiquitination of EZH2. J. Hematol. Oncol. 14(1), 196 (2021)
Y. Wang, J.H. Lu, Q.N. Wu, Y. **, D.S. Wang, Y.X. Chen et al., LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 18(1), 174 (2019)
B. Zhang, W. Bao, S. Zhang, B. Chen, X. Zhou, J. Zhao et al., LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 13(8), 734 (2022)
Funding
This study was funded by National Natural Science Foundation of China (No. 82073192 and 81773135).
Author information
Authors and Affiliations
Contributions
BW designed the study and supervised the process of research. HD, BC and ZQ performed the experiments, collected data, and wrote the original draft. HD and JG conducted the additional experiments during the revision phase. XX, HC, RZ, HL, TL, and JG analyzed and visualized the data. All authors contributed to this research and approved the final version.
Corresponding author
Ethics declarations
Ethics statement
The collection and analysis of clinical specimens were approved by Ethics Committee of Chinese PLA General Hospital. Informed consent was obtained from all included patients. The animal experiments were approved by Animal Center of Chinese PLA General Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Comparison of lncRNA CCAT2 expression between GC and adjacent normal tissues based on TCGA database. ***P < 0.001. (JPG 870 KB)
Supplementary Fig. 2
Overexpression of CD44v6 promotes proliferation, migration, invasion and EMT of GC cells. (A) WB analysis to show CD44v6 expression in cells that were stably infected with lentivirus carrying NC or CD44v6. (B) CCK-8 assay to show the proliferation capability of cells as in (A). (C) EdU assay to show the proliferation capability of cells as in (A). Scale bar: 100 μm. (D) Wound healing assay to show the migration capability of cells as in (A). (E) Transwell assay to show the invasion capability of cells as in (A). Scale bar: 100 μm. (F) WB analysis to show EMT-related proteins of cells as in (A). *P < 0.05. (JPG 7.38 MB)
Supplementary Fig. 3
Knockdown of ESRP1 decreased the ratio of CD44v6/CD44 expression. (JPG 307 KB)
Supplementary Fig. 4
ESRP2 does not participate in the regulation of alternative splicing of CD44v6. (JPG 361 KB)
Supplementary Fig. 5
Upregulation of ESRP1 expression facilitates malignant behaviors of GC cells. (A) WB analysis to show ESRP1 expression in cells that were stably infected with lentivirus carrying NC or ESRP1. (B) CCK-8 assay to show the proliferation capability of cells as in (A). (C) EdU assay to show the proliferation capability of cells as in (A). Scale bar: 100 μm. (D) Wound healing assay to show the migration capability of cells as in (A). (E) Transwell assay to show the invasion capability of cells as in (A). Scale bar: 100 μm. (F) WB analysis to show EMT-related proteins of cells as in (A). *P < 0.05, **P < 0.01. (JPG 7.12 MB)
ESM 1
(DOCX 20.7 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, H., Gao, J., Cao, B. et al. LncRNA CCAT2 promotes malignant progression of metastatic gastric cancer through regulating CD44 alternative splicing. Cell Oncol. 46, 1675–1690 (2023). https://doi.org/10.1007/s13402-023-00835-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00835-4