Abstract
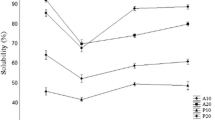
Protein isolate was prepared using demineralization and deproteinization of shrimp (Litopenaeus vannamei) shells (SSPI). SSPI was hydrolyzed via alcalase and papain at various concentrations (0.5–3%; w/v of protein) without and with ultrasonication pretreatment at 60 and 70% amplitude for 15 and 30 min. When SSPI was subjected to hydrolysis, the degree of hydrolysis (DH) was increased with increasing enzyme concentration, irrespective of the type of enzyme and ultrasonication pretreatment. DH of 43 and 31% was attained when 3% papain (UPH) and 2% alcalase (UAH), respectively, used along with pretreatment of ultrasonication at 60% amplitude for 15 min (p < 0.05). Higher protein solubility was noticed for UAH and UPH as compared to SSPI. UPH showed decreased foaming capacity (FC) and emulsifying properties than SSPI and UAH (p < 0.05), whereas 4% UAH had the highest FC than other samples (p < 0.05). Like UPH, UAH also showed lower emulsifying properties as compared to SSPI (p < 0.05). UAH had higher ABTS, and metal chelating activity as compared to UPH. On the other hand, UPH showed higher DPPH and FRAP activity than UAH. UPH and UAH were rich in hydrophilic amino acids and volatile compounds of different flavors. Both hydrolysates had peptides with a wide range of molecular weight. The bitterness increased when UAH and UPH levels increased higher than 6% (w/v). Thus, shrimp shell protein isolate could be exploited to produce protein hydrolysate of high DH with the help of ultrasonication pretreatment. Those protein isolates and hydrolysates could be implemented in several foods of several functional and antioxidant properties.




Similar content being viewed by others
References
Abuzar SHR, Sharif MK, Arshad R, Rehman A, Ashraf W, Karim A, Awan KA, Raza H, Khalid W, Asar TO, Al-Sameen MA (2023) Potential industrial and nutritional applications of shrimp by-products: a review. Int J Food Prop 26:3407–3432
Singh A, Benjakul S, Nuthong P, Prodpran T (2021) Elemental and structural changes associated with white spot formation in sun-dried Pacific white shrimp shells. Int J Food Sci Technol 56:2760–2767
Al Hoqani HAS, Noura A-S, Hossain MA, Al Sibani MA (2020) Isolation and optimization of the method for industrial production of chitin and chitosan from Omani shrimp shell. Carbohyd Res 492:108001
Lv J, Lv X, Ma M, Oh D-H, Jiang Z, Fu X (2023) Chitin and chitin-based biomaterials: A a review of advances in processing and food applications. Carbohyd Poly 299:120142
Pan I, Nanjundan K, Achuthan A, Issac PK, Rajagopal R, Chang SW, Bhat SA, Ravindran B (2023) Exploration of compost soil for the production of thermo-stable Bacillus protease to synthesize bioactive compounds through soy protein hydrolysis. Agronomy 13:1019
Wei M, Chen P, Zheng P, Tao X, Yu X, Wu D (2023) Purification and characterization of aspartic protease from Aspergillus niger and its efficient hydrolysis applications in soy protein degradation. Microb Cell Factories 22:42
Tawalbeh D, Ahmad WW, Sarbon N (2022) Effect of ultrasound pretreatment on the functional and bioactive properties of legumes protein hydrolysates and peptides: a comprehensive review. Food Rev Int 39:5423–5445
Alizadeh O, Aliakbarlu J (2020) Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. LWT-Food Sci Technol 131:109913
Xu Y, Yu J, Xue Y, Xue C (2023) Enhancing gel performance of surimi gels via emulsion co-stabilized with soy protein isolate and κ-carrageenan. Food Hydrocoll 135:108217
Kobayashi Y, Mayer SG, Park JW (2017) FT-IR and Raman spectroscopies determine structural changes of tilapia fish protein isolate and surimi under different comminution conditions. Food Chem 226:156–164
Li Y, Kong B, **a X, Liu Q, Li P (2013) Inhibition of frozen storage-induced oxidation and structural changes in myofibril of common carp ( Cyprinus carpio ) surimi by cryoprotectant and hydrolysed whey protein addition. Int J Food Sci Technol 48:1916–1923
Huang G, Ren Z, Jiang J (2011) Separation of iron-binding peptides from shrimp processing by-products hydrolysates. Food Bioproc Technol 4:1527–1532
Gómez-Estaca J, Albertos I, Martín-Diana AB, Rico D, Martínez-Álvarez Ó (2021) Protein hydrolysis and glycosylation as strategies to produce bioactive ingredients from unmarketable prawns. Foods 10:2844
AOAC (2002) Official Methods of Analysis, in Association of Official Analytical Chemists. DC, USA, Washington
Robinson HW, Hogden CG (1940) The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J Biol Chem 135:707–725
Ali M, Rawel H, Hellwig M (2023) Limited enzymatic hydrolysis of green coffee protein as a technique for preparing new functional food components. J Food Sci Technol 60:609–620
Inyang U, Iduh A (1996) Influence of pH and salt concentration on protein solubility, emulsifying and foaming properties of sesame protein concentrate. J Am Oil Chem Soc 73:1663–1667
Padial-Domínguez M, Espejo-Carpio FJ, Pérez-Gálvez R, Guadix A, Guadix EM (2020) Optimization of the emulsifying properties of food protein hydrolysates for the production of fish oil-in-water emulsions. Foods 9:636
Iglesias J, Medina I (2008) Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J Chromatogr A 1192:9–16
Deng J-J, Mao H-H, Fang W, Li Z-Q, Shi D, Li Z-W, Zhou T, Luo X-C (2020) Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J Clean Prod 271:122655
Liyanage C, Gonapinuwala S, Fernando C, De Croos M (2022) Physico-chemical properties of chitosan extracted from Whiteleg shrimp (Litopenaeus vannamei) processing shell waste in Sri Lanka. Sri Lanka J Aquat Sci 27:23–36
Puga Lopez D, Ponce Palafox JT, Barba Quintero G, Romero Beltran E, Garcia Ulloa Gomez M, Arredondo Figueroa JL, Torres Herrera MR (2013) Physicochemical, proximate composition, microbiological and sensory analysis of farmed and wild harvested white shrimp Litopenaeus vannamei (Bonne, 1931) tissues. Curr Res J Biol Sci 5:130–135
Ma H, Li Y, Liu D, Zhou H, Liu X, Wan Y, Zhao X (2023) Ultrasound-assisted limited enzymatic hydrolysis of high concentrated soy protein isolate: Alterations alterations on the functional properties and its relation with hydrophobicity and molecular weight. Ultrason Sonochem 95:106414
Walter C, Frieden E (1963) The prevalence and significance of the product inhibition of enzymes. Adv Enzymol Relat Subj Biochem 25:167–274
Tacias-Pascacio VG, Morellon-Sterling R, Siar E-H, Tavano O, Berenguer-Murcia Á, Fernandez-Lafuente R (2020) Use of alcalase in the production of bioactive peptides: a review. Int J Biol Macromol 165:2143–2196
Ozuna C, León-Galván MF (2017) Cucurbitaceae seed protein hydrolysates as a potential source of bioactive peptides with functional properties. BioMed Res Int 2017:2121878
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327
Ahmad Nadzri FN, Tawalbeh D, Sarbon NM (2021) Physicochemical properties and antioxidant activity of enzymatic hydrolysed chickpea (Cicer arietinum L.) protein as influence by alcalase and papain enzyme. Biocatal Agric Biotechnol 36:102131
Singh A, Benjakul S, Kishimura H (2017) Characteristics and functional properties of ovary from squid Loligo Formosana. J Aquat Food Prod Technol 26:1083–1092
Zayas JF (2012) Foaming properties of proteins. In Functionality of Proteins in Food (ed Zayas JF). Springer Science & Business Media, Berlin, Germany, pp 260–309
Mazorra-Manzano MA, Pacheco-Aguilar R, Ramírez-Suárez JC, Garcia-Sanchez G, Lugo-Sánchez ME (2012) Endogenous proteases in Pacific whiting (Merluccius productus) muscle as a processing aid in functional fish protein hydrolysate production. Food Bioproc Technol 5:130–137
Gbogouri G, Linder M, Fanni J, Parmentier M (2004) Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J Food Sci 69:C615–C622
Severin S, **a W (2006) Enzymatic hydrolysis of whey proteins by two different proteases and their effect on the functional properties of resulting protein hydrolysates. J Food Biochem 30:77–97
Tian Y, Pi J, Lv J, Chen Y, Ma M, Fu X (2024) The impact of ultrasound treatment combined with flaxseed gum on the foaming properties of egg white. Food Hydrocoll 148:109507
Lao Y, Ye Q, Wang Y, Vongsvivut J, Selomulya C (2023) Quantifying the effects of pre-roasting on structural and functional properties of yellow pea proteins. Food Res Int 172:113180
Boye J, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed S (2010) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Intl 43:537–546
dos Santos SDA, Martins VG, Salas-Mellado M, Prentice C (2011) Evaluation of functional properties in protein hydrolysates from bluewing searobin (Prionotus punctatus) obtained with different microbial enzymes. Food Bioproc Technol 4:1399–1406
Singh A, Putri GAU, Mittal A, Hong H, Yesilsu AF, Benjakul S (2021) Protein hydrolysate from splendid squid (Loligo formosana) fins: antioxidant, functional properties, and flavoring profile. Tur J Fish Aquat Sci 22:TRJFAS21005
Lin W-C, Chien J-T, Chen B-H (2005) Determination of carotenoids in spear shrimp shells (Parapenaeopsis hardwickii) by liquid chromatography. J Agric Food Chem 53:5144–5149
Sachindra NM, Mahendrakar NS (2011) Effect of protease treatment on oil extractability of carotenoids from shrimp waste. J Aquat Food Prod Technol 20:22–31
Wu H-C, Chen H-M, Shiau C-Y (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36:949–957
Nadzri FA, Tawalbeh D, Sarbon N (2021) Physicochemical properties and antioxidant activity of enzymatic hydrolysed chickpea (Cicer arietinum L.) protein as influence by alcalase and papain enzyme. Biocatal Agric Biotechnol 36:102131
Borrajo P, Pateiro M, Gagaoua M, Franco D, Zhang W, Lorenzo JM (2020) Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. App Sci 10:2290
O’Sullivan O’Sullivan SM, Lafarga T, Hayes M, O’Brien O’Brien NM (2017) Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and alcalase. J Food Biochem 41:e12406
Bah CS, Carne A, McConnell MA, Mros S, Bekhit AE-DA (2016) Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem 202:458–466
Tkaczewska J, Borawska-Dziadkiewicz J, Kulawik P, Duda I, Morawska M, Mickowska B (2020) The effects of hydrolysis condition on the antioxidant activity of protein hydrolysate from Cyprinus carpio skin gelatin. LWT-Food Sci Technol 117:108616
Ou K, Liu Y, Zhang L, Yang X, Huang Z, Nout MJR, Liang J (2010) Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 cells. J Agric Food Chem 58:4894–4900
Kim HO, Li-Chan ECY (2006) Quantitative structure-activity relationship study of bitter peptides. J Agric Food Chem 54:10102–10111
Maehashi K, Huang L (2009) Bitter peptides and bitter taste receptors. Cell Mol Life Sci 66:1661–1671
Du Q, Tu M, Liu J, Ding Y, Zeng X, and Pan D (2023) Plant-based meat analogs and fat substitutes, structuring technology and protein digestion: a review. Food Res Int 112959. https://doi.org/10.1016/j.foodres.2023.112959
Iwasaki M, Harada R (1985) Proximate and amino acid composition of the roe and muscle of selected marine species. J Food Sci 50:1585–1587
Zhang J, Pavlova NN, Thompson CB (2017) Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. The EMBO J 36:1302–1315
Okabe Y, Inoue Y, Kanda Y, Katsumata T (2019) Odor-active compounds contributing to the characteristic aroma of shrimp cooked whole, including shells and viscera. Euro Food Res Technol 245:233–241
Rochat S, Egger J, Chaintreau A (2009) Strategy for the identification of key odorants: application to shrimp aroma. J Chromatogr A 1216:6424–6432
Jovanović JR, Stefanović AB, Šekuljica NŽ, Tanasković SMJ, Dojčinović MB, Bugarski BM, Knežević‐Jugović ZD (2016) Ultrasound pretreatment as an useful tool to enhance egg white protein hydrolysis: kinetics, reaction model, and thermodinamics. J Food Sci 81:C2664–C2675
Fadimu GJ, Gill H, Farahnaky A, Truong T (2021) Investigating the impact of ultrasound pretreatment on the physicochemical, structural, and antioxidant properties of lupin protein hydrolysates. Food Bioproc Technol 14:2004–2019
Acknowledgments
This research acknowledged the space and support provided by the Prince of Songkla University (PSU). Support from Prachayacharn grant from PSU was also acknowledged.
Funding
This research was supported by the National Science, Research, and Innovation Fund (NSRF) and Prince of Songkla University (PSU) (grant number AGR6701323S and AGR6601347S).
Author information
Authors and Affiliations
Contributions
Conceptualization: Avtar Singh and Soottawat Benjakul. Methodology: Akanksha R Gautam. Software: Akanksha R. Gautam and Ajay Mittal. Validation: Avtar Singh and Soottawat Benjakul. Formal analysis: Akanksha R. Gautam. Investigation: Akanksha R. Gautam and Ajay Mittal. Resources: Avtar Singh and Soottawat Benjakul. Data curation: Avtar Singh and Prabjeet Singh. Writing—original draft preparation: Akanksha R. Gautam. Writing—review and editing: Soottawat Benjakul and Prabjeet Singh. Visualization: Akanksha R. Gautam and Avtar Singh. Supervision: Avtar Singh. Project administration: Avtar Singh. Funding acquisition: Avtar Singh and Soottawat Benjakul. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gautam, A., Benjakul, S., Mittal, A. et al. Ultrasonication-assisted enzymatic hydrolysis of shrimp shell protein isolate: characterization, antioxidant, and functional properties. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05637-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05637-8