Abstract
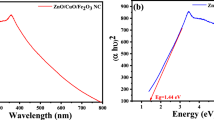
The growing concern over wastewater pollution caused by hazardous organic dyes has necessitated the search for eco-friendly and efficient methods to combat dye contamination. Nanotechnology has emerged as a promising solution, with photocatalysis playing a vital role in organic pollutant degradation. This study presents a novel and eco-friendly approach to the green synthesis of α-Fe2O3@Ag nanocomposites (NCs) using Citrus sinensis leaf extract as a biocompatible reducing agent. The synthesized NCs were characterized using various techniques, including UV–vis spectrometry, Fourier transform infrared spectroscopy, and X-ray diffraction. We explore their potential applications in the degradation of rose Bengal dye and antimicrobial activity. The synthesized α-Fe2O3 nanoparticles (NPs) and α-Fe2O3@Ag NCs exhibited a spherical and cubical morphology with an average particle size of 50 nm. Furthermore, the average crystallite sizes were determined to be 25.65 nm for α-Fe2O3 NPs and 23.53 nm for α-Fe2O3@Ag. Notably, the optical band gap energy of α-Fe2O3 NPs and α-Fe2O3@Ag NCs was calculated to be 1.94 and 1.25 eV, respectively. The α-Fe2O3@Ag NCs exhibit superior photocatalytic efficiency, achieving a degradation rate of 95.15% within 60 min under sunlight exposure, outperforming α-Fe2O3 NPs (90.99%). The NC demonstrates strong antimicrobial activity against various bacterial strains, with inhibition zones ranging from 15 to 19 mm at 20 mg/mL concentration. Moreover, both α-Fe2O3@Ag NCs and α-Fe2O3 NPs exhibit anti-candidiasis activity against Candida albicans. The findings underscore the promising potential of α-Fe2O3@Ag NCs as sustainable and efficient solutions for dye degradation and antimicrobial activity, offering a greener approach to nanomaterial synthesis and environmental remediation.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bhavya G et al (2021) Remediation of emerging environmental pollutants: a review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 275:129975
Stevens SY, Sutherland LM, Krajcik JS (2009) The big ideas of nanoscale science and engineering. NSTA press.
Klabunde KJ, Richards RM (2009) Nanoscale materials in chemistry. John Wiley & Sons.
Fréchet JM (2003) Dendrimers and other dendritic macromolecules: from building blocks to functional assemblies in nanoscience and nanotechnology. J Polym Sci Part A: Polym Chem 41(23):3713–3725
Roco M (1999) Towards a US national nanotechnology initiative. J Nanopart Res 1(4):435
Mauter MS, Elimelech M (2008) Environmental applications of carbon-based nanomaterials. Environ Sci Technol 42(16):5843–5859
Obare SO, Meyer GJ (2004) Nanostructured materials for environmental remediation of organic contaminants in water. J Environ Sci Health Part A 39(10):2549–2582
Samadi Z et al (2021) Facile green synthesis of zero-valent iron nanoparticles using barberry leaf extract (GnZVI@ BLE) for photocatalytic reduction of hexavalent chromium. Bioorg Chem 114:105051
Patiño-Ruiz D et al (2020) Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: nanotoxicological considerations for potential environmental applications. Environ Nanotechnol Monitor Manag 14:100377
Ardakani LS et al (2021) Green synthesis of iron-based nanoparticles using Chlorophytum comosum leaf extract: methyl orange dye degradation and antimicrobial properties. Heliyon 7(2)
Charbgoo F, Ahmad MB, Darroudi M (2017) Cerium oxide nanoparticles: green synthesis and biological applications. Int J Nanomed 1401–1413
Ijaz I et al (2020) Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev 13(3):223–245
González-Jiménez EE (2016) Control de la superficie y el volumen en la nanoescala para la configuración y el diseño de nanodispositivos. Rev Acad Colomb Cienc Exactas Físicas Nat 40(157):590–599
Tadic M et al (2021) Rhombohedron and plate-like hematite (α-Fe2O3) nanoparticles: synthesis, structure, morphology, magnetic properties and potential biomedical applications for MRI. Mater Res Bull 133:111055
Abdullah JAA et al (2022) Green synthesis of FexOy nanoparticles with potential antioxidant properties. Nanomaterials 12(14):2449
Zhang X et al (2013) Synthesis, optical and magnetic properties of α-Fe2O3 nanoparticles with various shapes. Mater Lett 99:111–114
Hasan GG et al (2023) Synergistic effect of novel biosynthesis SnO2@ Fe3O4 nanocomposite: a comprehensive study of its photocatalytic of dyes & antibiotics, antibacterial, and antimutagenic activities. J Photochem Photobiol A 443:114874
Rita A et al (2019) Shock wave recovery studies on structural and magnetic properties of α—Fe2O3 NPs. Mater Res Express 6(9):095035
Irshad MA et al (2021) Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: a review. Ecotoxicol Environ Saf 212:111978
Mughal SS, Hassan SM (2022) Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: a review. Am J Mater Synth Process 7(2):15–28
Soufi GJ, Iravani S (2020) Eco-friendly and sustainable synthesis of biocompatible nanomaterials for diagnostic imaging: current challenges and future perspectives. Green Chem 22(9):2662–2687
Duan H, Wang D, Li Y (2015) Green chemistry for nanoparticle synthesis. Chem Soc Rev 44(16):5778–5792
Oliveira JLD et al (2018) Zein nanoparticles as eco-friendly carrier systems for botanical repellents aiming sustainable agriculture. J Agric Food Chem 66(6):1330–1340
Muthoosamy K et al (2015) Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int J Nanomed 1505–1519
Maurya IC et al (2019) Green synthesis of TiO2 nanoparticles using Bixa orellana seed extract and its application for solar cells. Sol Energy 194:952–958
Islam F et al (2022) Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 15(6):2160
Fierascu RC et al (2019) Phyto-nanocatalysts: green synthesis, characterization, and applications. Molecules 24(19):3418
Saratale RG et al (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res 25:10164–10183
Yadav M et al (2022) Biogenic silica nanoparticles from agro-waste: properties, mechanism of extraction and applications in environmental sustainability. J Environ Chem Eng 108550
Mulvihill MJ et al (2011) Green chemistry and green engineering: a framework for sustainable technology development. Annu Rev Environ Resour 36:271–293
Raja RK et al (2022) Green nanotechnology for the environment. Handbook of Microbial Nanotechnology. Elsevier, pp 461–478
Alheshibri M et al (2022) Synthesis of Ag nanoparticles-decorated on CNTs/TiO2 nanocomposite as efficient photocatalysts via nanosecond pulsed laser ablation. Opt Laser Technol 155:108443
Al Baroot A et al (2022) A novel approach for fabrication ZnO/CuO nanocomposite via laser ablation in liquid and its antibacterial activity. Arab J Chem 15(2):103606
Al Baroot A et al (2023) Efficient catalytic reduction of 2-nitrophenol using cellulose acetate butyrate/CuO nanocomposite prepared by laser ablation technique. J Polym Environ 1–12
Manda AA et al (2023) Fast one-pot laser-based fabrication of ZnO/TiO2-reduced graphene oxide nanocomposite for photocatalytic applications. Opt Laser Technol 160:109105
Al Baroot A et al (2023) Photocatalytic performance of Ag/TiO2/SiO2 nanocomposite synthesized by eco-friendly pulsed laser ablation technique. J Phys Chem Solids, 111489
Manda AA et al (2023) Catalytic activity of cellulose acetate butyrate/TiO2-Au nanocomposite film prepared by laser ablation for 2-nitrophenol reduction. J Polym Environ. 1–12
Kir I et al (2023) Biosynthesis and characterization of novel nanocomposite ZnO/BaMg2 efficiency for high-speed adsorption of AZO dye. Biomass Convers Biorefinery. 1–10
Rani N et al (2023) Plant-mediated synthesis of nanoparticles and their applications: a review. Mater Res Bull 112233
Ahmed S et al (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28
Shah Y, Maharana M, Sen S (2022) Peltophorum pterocarpum leaf extract mediated green synthesis of novel iron oxide particles for application in photocatalytic and catalytic removal of organic pollutants. Biomass Convers Biorefinery 1–14
Restrepo CV, Villa CC (2021) Synthesis of silver nanoparticles, influence of cap** agents, and dependence on size and shape: a review. Environ Nanotechnol Monit Manag 15:100428
Dutta AK, Maji SK, Adhikary B (2014) γ-Fe2O3 nanoparticles: an easily recoverable effective photo-catalyst for the degradation of rose Bengal and methylene blue dyes in the waste-water treatment plant. Mater Res Bull 49:28–34
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Biores Technol 97(9):1061–1085
Pereira L, Alves M (2012) Dyes—environmental impact and remediation. Environmental protection strategies for sustainable development, 111–162.
Nandhini N, Rajeshkumar S, Mythili S (2019) The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal Agric Biotechnol 19:101138
Ghannoum MA, Rice LB (1999) Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12(4):501–517
Navya P, Daima HK (2016) Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Convergence 3:1–14
Abdallah BM, Ali EM (2021) Green synthesis of silver nanoparticles using the Lotus lalambensis aqueous leaf extract and their anti-candidal activity against oral candidiasis. ACS Omega 6(12):8151–8162
Bautista-Guzman J et al (2021) Influence of the alcoholic/ethanolic extract of mangifera indica residues on the green synthesis of FeO nanoparticles and their application for the remediation of agricultural soils. Molecules 26(24):7633
Tauc J, Menth A (1972) States in the gap. J Non-Cryst Solids 8:569–585
Hmamouchi S, El Yacoubi A, El Idrissi BC (2022) Using egg ovalbumin to synthesize pure α-Fe<sub>2</sub>O<sub>3</sub> and cobalt doped α-Fe<sub>2</sub>O<sub>3</sub>: structural, morphological, optical and photocatalytic properties. Heliyon 8(2)
Bouafia A et al (2023) Removal of hydrocarbons and heavy metals from petroleum water by modern green nanotechnology methods. Sci Rep 13(1):5637
Muthukumar H et al (2020) Plant extract mediated synthesis enhanced the functional properties of silver ferrite nanoparticles over chemical mediated synthesis. Biotechnol Rep 26:e00469
Ramesh A et al (2018) Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract of Zanthoxylum armatum DC. for efficient adsorption of methylene blue. J Asian Ceram Soc 6(2):145–155
Yi Y et al (2019) Green synthesis of iron-based nanoparticles from extracts of Nephrolepis auriculata and applications for Cr (VI) removal. Mater Lett 234:388–391
Dhar PK et al Cleaner engineering and technology
Sathishkumar G et al (2018) Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif Cells Nanomed Biotechnol 46(3):589–598
Cornell R et al (2004) Colour, the iron oxides. Wiley, New York
Zachariasen WH, Skr. Norske Vidensk. Akad. Oslo, I. Mat. Naturvidensk. Kl, 1928. 4.
Bouafia A, Laouini SE (2020) Green synthesis of iron oxide nanoparticles by aqueous leaves extract of Mentha Pulegium L.: effect of ferric chloride concentration on the type of product. Mater Lett 265:127364
Owen EA, Williams GI (1954) A low-temperature X-ray camera. J Sci Instrum 31(2):49
Mohammed Mohammed HA et al (2023) A novel biosynthesis of MgO/PEG nanocomposite for organic pollutant removal from aqueous solutions under sunlight irradiation. Environ Sci Pollut Res 1–10
Mohammed Mohammed HA et al (2023) A novel biosynthesis of MgO/PEG nanocomposite for organic pollutant removal from aqueous solutions under sunlight irradiation. Environ Sci Pollut Res 30(19):57076–57085
Mohammed HA et al (2023) Green synthesis of SnO2 nanoparticles from Laurus nobilis L. extract for enhanced gelatin-based films and CEF@ SnO2 for efficient antibacterial activity. Food Bioprocess Technol 1–19
Čapek J, Roušar T (2021) Detection of oxidative stress induced by nanomaterials in cells—the roles of reactive oxygen species and glutathione. Molecules 26(16):4710
Mammari N et al (2022) Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 10(2):437
Bahrami A et al (2022) Targeting foodborne pathogens via surface-functionalized nano-antimicrobials. Adv Coll Interface Sci 302:102622
Singh A et al (2020) Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol Rep 25:e00427
Barros D et al (2021) Transcriptomics reveals the action mechanisms and cellular targets of citrate-coated silver nanoparticles in a ubiquitous aquatic fungus. Environ Pollut 268:115913
Khina A, Krutyakov YA (2021) Similarities and differences in the mechanism of antibacterial action of silver ions and nanoparticles. Appl Biochem Microbiol 57:683–693
Zhang Y et al (2020) Influence of the spatial distribution of cationic functional groups at nanoparticle surfaces on bacterial viability and membrane interactions. J Am Chem Soc 142(24):10814–10823
Saha B et al (2011) Preferential and enhanced adsorption of different dyes on iron oxide nanoparticles: a comparative study. J Phys Chem C 115(16):8024–8033
Dehno Khalaji A (2022) Spherical α Fe2O3 nanoparticles: synthesis and characterization and its photocatalytic degradation of methyl orange and methylene blue. Phys Chem Res 10(4):473–483
Das B, Jena S, Dhal J (2021) Ag doped α-Fe2O3 nanoparticles: synthesis, characterization and application as heterogeneous photocatalyst for removal of organic dye from aqueous media without any oxidizing agents. J Indian Chem Soc 98(11):100214
Acknowledgements
The authors would like to thank the Laboratory of Biotechnology Biomaterials and Condensed Matter at the University of El Oued, Algeria, and the Researchers Supporting Project (RSP2023R160), King Saud University (Riyadh, Saudi Arabia).
Author information
Authors and Affiliations
Contributions
Conceptualization, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., J.A.A.A., and F.A.; data curation, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., J.A.A.A., and F.A.; formal analysis, R.Z.A., A.B., S.M., and F.A.; investigation, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., J.A.A.A., and F.A.; methodology, R.Z.A., S.E.L., C.S., A.B., S.M., and F.A.; project administration, R.Z.A., S.E.L., S.C., A.B., S.M., H.A.M., C.S., and F.A.; resources, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., J.A.A.A., and F.A.; software, R.Z.A., A.B., S.M., and F.A.; supervision, S.M., A.B., S.E.L.; validation, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., and F.A.; visualization, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., and F.A.; writing — original draft, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., and F.A.; writing — review and editing, R.Z.A., S.E.L., C.S., A.B., S.M., H.A.M., S.C., and F.A.; the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zouari Ahmed, R., Laouini, S.E., Salmi, C. et al. Green synthesis of α-Fe2O3 and α-Fe2O3@Ag NC for degradation of rose Bengal and antimicrobial activity. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05046-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05046-3