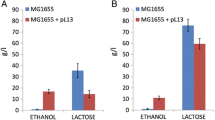
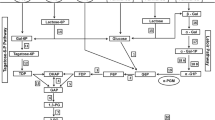
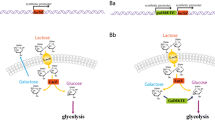
Abstract
Global milk production has increased by 59% since 1988, reaching 944 million tonnes in 2023. This surge has resulted in an annual by-product, whey, totalling 200 million tonnes. Despite half of this whey being utilized in the food and pharmaceutical sectors, significant quantities are discarded, posing environmental challenges due to its high biological oxygen demand. Whey proteins and lipids are recovered from whey, and the resultant whey permeate stream is rich in lactose is a viable carbon source for value-added biochemical. D(-)lactic acid (DLA) is a versatile organic acid molecule widely used in the synthesis of thermostable biodegradable polymers viz., polylactic acid (PLA). However, the DLA market faces constraints; downstream demand is unsaturated, and upstream production is restricted, leading to elevated costs and posing challenges to its growth and profitability, which affect PLA manufacturing. This review study addresses a novel techno-economic approach involving strategies for genetic engineering of lactic acid bacteria and process intensification towards efficient valorization of whey permeate into DLA. This review enumerates a circular bioeconomy approach offering both environmental benefits and revenue opportunities for the dairy sector, revolutionizing by-product utilization and enhancing the industry’s sustainability.









Similar content being viewed by others
Abbreviations
- BOD:
-
Biological oxygen demand
- BSA:
-
Bovine serum albumin
- CAGR:
-
Compound annual growth rate
- COD:
-
Chemical oxygen demand
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- DLA:
-
D-lactic acid
- FDA:
-
Food and Drug Administration
- GRAS:
-
Generally Recognized as Safe
- HFLAB:
-
Homofermentative lactic acid bacteria
- HPLC:
-
High-performance liquid chromatography
- IgG:
-
Immunoglobulins
- LAB:
-
Lactic acid bacteria
- Ldh:
-
Lactate dehydrogenase
- LF:
-
Lactoferrin
- LPO:
-
Lactoperoxidase
- MPC:
-
Milk protein concentrate
- MWCO:
-
Molecular weight cutoff
- NAD:
-
Nicotinamide adenine dinucleotide
- NADH:
-
Nicotinamide adenine dinucleotide (NAD) + hydrogen (H)
- NCBI:
-
National Centre for Biotechnology Information
- NIRS:
-
Near-infrared spectroscopy
- MS:
-
Mass spectrometry
- PDLA:
-
Poly-D-lactic acid
- PLA:
-
Polylactic acid
- PLLA:
-
Poly-L-lactic acid
- SCP:
-
Single cell protein
- SMP:
-
Skim milk powder
- UF:
-
Ultrafiltration
- USD:
-
United States dollar
References
Singh DP, Dwevedi A (2019) Production of clean energy by green ways. Solutions to Environmental Problems Involving Nanotechnology and Enzyme Technology, pp 49–90
Guddaraddi A, Singh A, Amrutha G et al (2023) Sustainable biofuel production from agricultural residues an eco-friendly approach: a review. Int J Environ Clim Change 13:2905–2914
Ahmad T, Aadil RM, Ahmed H et al (2019) Treatment and utilization of dairy industrial waste: a review. Trends Food Sci Technol 88:361–372
Pilarska AA, Pilarski K (2023) Bioenergy generation from different types of waste by anaerobic digestion. Energies 16(16):6919. https://doi.org/10.3390/EN16196919
Permana I, Sudirja R, Jupesta J (2023) Potential of vermifiltration technique to reduce chemical oxygen demand, biological oxygen demand, and total suspended solid of farm dairy effluent in develo** countries: case of Indonesian farm dairy industry. Green and Low-Carbon Economy. https://doi.org/10.47852/BONVIEWGLCE3202933
Espíndola JC, Mierzwa JC, Amaral MCS, De Andrade LH (2023) Water reuse through membrane technologies for a dairy plant using water pinch simulation software. Sustainability 15:2540. https://doi.org/10.3390/su15032540
Zotta T, Solieri L, Iacumin L et al (2020) Valorization of cheese whey using microbial fermentations. Appl Microbiol Biotechnol 104:2749–2764
Lappa IK, Papadaki A, Kachrimanidou V et al (2019) Cheese whey processing: integrated biorefinery concepts and emerging food applications. Foods 8:347
Eskandar K (2023) Revolutionizing biotechnology and bioengineering: unleashing the power of innovation. Artic J Appl Biotechnol Bioeng. https://doi.org/10.15406/jabb.2023.10.00332
Chandra R, Castillo-Zacarias C, Delgado P, Parra-Saldívar R (2018) A biorefinery approach for dairy wastewater treatment and product recovery towards establishing a biorefinery complexity index. J Clean Prod 183:1184–1196
Shete BS, Shinkar NP (2013) Dairy industry wastewater sources, characteristics & its effects on environment. Int J Curr Eng Technol 3:1611–1615
Arvanitoyannis IS, Giakoundis A (2006) Current strategies for dairy waste management: a review. Crit Rev Food Sci Nutr 46:379–390
Kushwaha JP, Srivastava VC, Mall ID (2011) An overview of various technologies for the treatment of dairy wastewaters. Crit Rev Food Sci Nutr 51:442–452
Slavov AK (2017) Dairy wastewaters–general characteristics and treatment possibilities—a review. Food Technol Biotechnol 55:14–28
Roufou S, Griffin S, Katsini L et al (2021) The (potential) impact of seasonality and climate change on the physicochemical and microbial properties of dairy waste and its management. Trends Food Sci Technol 116(2021):1–10
Joshiba GJ, Kumar PS, Femina CC et al (2019) Critical review on biological treatment strategies of dairy wastewater. Desalination Water Treat 160:94–109
Goli A, Shamiri A, Khosroyar S et al (2019) A review on different aerobic and anaerobic treatment methods in dairy industry wastewater. J Environ Treat Tech 6:113–141
Porwal HJ, Mane AV, Velhal SG (2015) Biodegradation of dairy effluent by using microbial isolates obtained from activated sludge. Water Resour Ind 9:1–15
Danalewich JR, Papagiannis TG, Belyea RL et al (1998) Characterization of dairy waste streams, current treatment practices, and potential for biological nutrient removal. Water Res 32:3555–3568. https://doi.org/10.1016/S0043-1354%2898%2900160-2
Ryan MP, Walsh G (2016) The biotechnological potential of whey. Rev Environ Sci Biotechnol 15:479–498
Birwal P, Deshmukh G, Priyanka SSP, Saurabh SP (2017) Advanced technologies for dairy effluent treatment. J Food, Nutr Popul Health 1:7
Samudro G, Mangkoedihardjo S (2010) Review on BOD, COD and BOD/COD ratio: a triangle zone for toxic, biodegradable and stable levels. Int J Acad Res 2
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445:385–396
Alsaed AK, Ahmad R, Aldoomy H et al (2013) Characterization, concentration and utilization of sweet and acid whey. Pakistan J Nutr 12:172
Prazeres AR, Carvalho F, Rivas J (2012) Cheese whey management: a review. J Environ Manage 110:48–68
O’Regan J, Ennis MP, Mulvihill DM (2009) Milk proteins. In: Handbook of hydrocolloids. Elsevier, pp 298–358
Pires AF, Marnotes NG, Rubio OD et al (2021) Dairy by-products: a review on the valorization of whey and second cheese whey. Foods 10:1067
León-López A, Pérez-Marroquín XA, Estrada-Fernández AG et al (2022) Milk whey hydrolysates as high value-added natural polymers: functional properties and applications. Polymers 14(14):1258. https://doi.org/10.3390/POLYM14061258
Ha E, Zemel MB (2003) Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people. J Nutr Biochem 14:251–258
Krissansen GW (2007) Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr 26:713S–723S
Park YW, Nam MS (2015) Bioactive peptides in milk and dairy products: a review. Korean J Food Sci Anim Resour 35:831
Chung C, Degner B, McClements DJ (2014) Development of reduced-calorie foods: microparticulated whey proteins as fat mimetics in semi-solid food emulsions. Food Res Int 56:136–145
Ipsen R (2017) Microparticulated whey proteins for improving dairy product texture. Int Dairy J 67:73–79
Jovanović S, Barać M, Maćej O (2005) Whey proteins-properties and possibility of application. Mljekarstvo: Časopis za Unaprjeđenje Proizvodnje i Prerade Mlijeka 55:215–233
Stanchev P, Vasilaki V, Egas D et al (2020) Multilevel environmental assessment of the anaerobic treatment of dairy processing effluents in the context of circular economy. J Clean Prod 261:121139
Sanmartín B, Díaz O, Rodríguez-Turienzo L, Cobos A (2012) Composition of caprine whey protein concentrates produced by membrane technology after clarification of cheese whey. Small Rumin Res 105:186–192
Pedersen L, Mollerup J, Hansen E, Jungbauer A (2003) Whey proteins as a model system for chromatographic separation of proteins. J Chromatogr B 790:161–173
Kumar R, Chauhan SK, Shinde G et al (2018) Whey proteins: a potential ingredient for food industry—a review. Asian J Dairy Food Res 37:283–290
Chavan RS, Shraddha RC, Kumar A, Nalawade T (2015) Whey based beverage: its functionality, formulations, health benefits and applications. J Food Process Technol 6:1
Jelen P (2009) Whey-based functional beverages. In: Functional and speciality beverage technology. Elsevier, pp 259–280
JK AT (2019) Logomaker: beautiful sequence logos in python. Bioinformatics 36:2272–2274. https://doi.org/10.1093/bioinformatics/btz921
Stobaugh HC, Ryan KN, Kennedy JA et al (2016) Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial. Am J Clin Nutr 103:926–933
Baldasso C, Barros TC, Tessaro IC (2011) Concentration and purification of whey proteins by ultrafiltration. Desalination 278:381–386
Spalatelu C (2012) Biotechnological valorisation of whey. Innov Rom Food Biotechnol 10:1
de Souza RR, Bergamasco R, da Costa SC et al (2010) Recovery and purification of lactose from whey. Chem Eng Process: Process Intensif 49:1137–1143
Singh DP, Dwevedi A (2018) Production of clean energy by green ways. Solutions to Environmental Problems Involving Nanotechnology and Enzyme Technology, pp 49–90. https://doi.org/10.1016/B978-0-12-813123-7.00002-5
Hargrove RE, McDonough FE, Lacroix DE, Alford JA (1976) Production and properties of deproteinized whey powders. J Dairy Sci 59:25–33
Cuartas-Uribe B, Alcaina-Miranda MI, Soriano-Costa E et al (2009) A study of the separation of lactose from whey ultrafiltration permeate using nanofiltration. Desalination 241:244–255
Listiohadi YD, Hourigan JA, Sleigh RW, Steele RJ (2005) An exploration of the caking of lactose in whey and skim milk powders. Aust J Dairy Technol 60:207
Yang S-T, Silva EM (1995) Novel products and new technologies for use of a familiar carbohydrate, milk lactose. J Dairy Sci 78:2541–2562
Gupta C, Prakash D (2017) Therapeutic potential of milk whey. Beverages 3:31
Fox PF (1997) Lactose, water, salts and vitamins. Adv Dairy Chem 3:536
Sar T, Stark BC, Akbas MY (2019) Bioethanol production from whey powder by immobilized E. coli expressing Vitreoscilla hemoglobin: optimization of sugar concentration and inoculum size. Biofuels
Jayamuthunagai J, Srisowmeya G, Chakravarthy M, Gautam P (2017) D-Tagatose production by permeabilized and immobilized Lactobacillus plantarum using whey permeate. Bioresour Technol 235:250–255
Grenov B, Briend A, Sangild PT et al (2016) Undernourished children and milk lactose. Food Nutr Bull 37:85–99
Zadow JG (1984) Lactose: properties and uses. J Dairy Sci 67:2654–2679
Nagar S, Nagal S (2013) Whey: composition, role in human health and its utilization in preparation of value added products. Int J Food Ferment Technol 3:93–100
Turner TL, Kim E, Hwang C et al (2017) Conversion of lactose and whey into lactic acid by engineered yeast. J Dairy Sci 100:124–128
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Guimarães PMR, Teixeira JA, Domingues L (2010) Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv 28:375–384
Ghaly AE, Kamal M, Correia LR (2005) Kinetic modelling of continuous submerged fermentation of cheese whey for single cell protein production. Bioresour Technol 96:1143–1152
Mahmoud MM, Kosikowski FV (1982) Alcohol and single cell protein production by Kluyveromyces in concentrated whey permeates with reduced ash. J Dairy Sci 65:2082–2087
Barrett E, Stanton C, Zelder O et al (2004) Heterologous expression of lactose-and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey. Appl Environ Microbiol 70:2861–2866
Büyükkileci AO, Harsa S (2004) Batch production of L (+) lactic acid from whey by Lactobacillus casei (NRRL B-441). J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 79:1036–1040
Panesar PS, Kennedy JF, Knill CJ, Kosseva M (2010) Production of L (+) lactic acid using Lactobacillus casei from whey. Braz Arch Biol Technol 53:219–226
Gandhi DN, Patel RS, Wadhwa BK et al (2000) Effect of agro-based by-products on production of lactic acid in whey permeate medium. J Food Sci Technol (Mysore) 37:292–295
Sahoo TK, Jayaraman G (2019) Co-culture of Lactobacillus delbrueckii and engineered Lactococcus lactis enhances stoichiometric yield of d-lactic acid from whey permeate. Appl Microbiol Biotechnol 103:5653–5662
Prasad S, Srikanth K, Limaye AM, Sivaprakasam S (2014) Homo-fermentative production of d-lactic acid by Lactobacillus sp. employing casein whey permeate as a raw feed-stock. Biotechnol Lett 36:1303–1307
Reddy Tadi SR, EVR A, Limaye AM, Sivaprakasam S (2017) Enhanced production of optically pure D (–) lactic acid from nutritionally rich Borassus flabellifer sugar and whey protein hydrolysate based–fermentation medium. Biotechnol Appl Biochem 64:279–289
Liu P, Zheng Z, Xu Q et al (2018) Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochem 71:18–22
Gali KK, Mukherjee P, Katiyar V, Sivaprakasam S (2022) Process efficacy in cassava-based biorefinery: scalable process technology for the development of green monomer d-lactic acid, pp 107–134. https://doi.org/10.1007/978-981-19-4316-4_5
Mehta R, Kumar V, Bhunia H, Upadhyay SN (2005) Synthesis of poly (lactic acid): a review. J Macromol Sci C: Polym Rev 45:325–349
Jia S, Yu D, Zhu Y et al (2017) Morphology, crystallization and thermal behaviors of PLA-based composites: wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers (Basel) 9:528
Klotz S, Kaufmann N, Kuenz A, Prüße U (2016) Biotechnological production of enantiomerically pure d-lactic acid. Appl Microbiol Biotechnol 100:9423–9437
Lebarbe T (2013) Synthesis of novel “green” polyesters from plant oils: application to the rubber-toughening of poly (L-lactide)(Doctoral dissertation, Université Sciences et Technologies-Bordeaux I)
Fernandes MC, Alves-Ferreira J, Duarte LC et al (2023) D-lactic acid production from hydrothermally pretreated, alkali delignified and enzymatically saccharified rockrose with the metabolic engineered Escherichia coli strain JU15. Biomass Convers Biorefin 13:12849–12858. https://doi.org/10.1007/S13399-021-02207-0/TABLES/5
Arshadi M, Attard TM, Lukasik RM et al (2016) Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem 18:6160–6204
Yamada R, Yokota M, Matsumoto T et al (2023) Promoting cell growth and characterizing partial symbiotic relationships in the co-cultivation of green alga Chlamydomonas reinhardtii and Escherichia coli. Biotechnol J 18:2200099. https://doi.org/10.1002/BIOT.202200099
Ahmad A, Banat F, Taher H (2020) A review on the lactic acid fermentation from low-cost renewable materials: recent developments and challenges. Environ Technol Innov 101138
Mimitsuka T, Sawai K, Kobayashi K et al (2015) Production of d-lactic acid in a continuous membrane integrated fermentation reactor by genetically modified Saccharomyces cerevisiae: enhancement in d-lactic acid carbon yield. J Biosci Bioeng 119:65–71
Beitel SM, Coelho LF, Contiero J (2020) Efficient conversion of agroindustrial waste into D (-) lactic acid by Lactobacillus delbrueckii using fed-batch fermentation. Biomed Res Int 2020
Bai Z, Gao Z, Sun J et al (2016) D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresour Technol 207:346–352
Ahring BK, Traverso JJ, Murali N, Srinivas K (2016) Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem Eng J 109:162–169
Abdel-Rahman MA, Tashiro Y, Zendo T et al (2016) Highly efficient L-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25. RSC Adv 6:17659–17668
Lee HD, Lee MY, Hwang YS et al (2017) Separation and purification of lactic acid from fermentation broth using membrane-integrated separation processes. Ind Eng Chem Res 56:8301–8310
Komesu A, de Oliveira JAR, da Silva Martins LH et al (2017) Lactic acid production to purification: a review. Bioresources 12:4364–4383
Su C-Y, Yu C-C, Chien I-L, Ward JD (2015) Control of highly interconnected reactive distillation processes: purification of raw lactic acid by esterification and hydrolysis. Ind Eng Chem Res 54:6932–6940
Komesu A, Wolf Maciel MR, Rocha de Oliveira JA et al (2017) Purification of lactic acid produced by fermentation: focus on non-traditional distillation processes. Sep Purif Rev 46:241–254
Wang L, Cai Y, Zhu L et al (2014) Major role of NAD-dependent lactate dehydrogenases in the production of l-lactic acid with high optical purity by the thermophile Bacillus coagulans. Appl Environ Microbiol 80:7134–7141
Mazzoli R, Bosco F, Mizrahi I et al (2014) Towards lactic acid bacteria-based biorefineries. Biotechnol Adv 32:1216–1236
van de Guchte M, Penaud S, Grimaldi C et al (2006) The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc Nat Acad Sci 103:9274–9279
Yadav M, Shukla P (2020) Efficient engineered probiotics using synthetic biology approaches: a review. Biotechnol Appl Biochem 67:22–29
Manome A, Okada S, Uchimura T, Komagata K (1998) The ratio of L-form to D-form of lactic acid as a criteria for the identification of lactic acid bacteria. J Gen Appl Microbiol 44:371–374
Tanaka T, Hoshina M, Tanabe S et al (2006) Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour Technol 97:211–217
Calabia BP, Tokiwa Y (2007) Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnol Lett 29:1329–1332
Yáñez R, Moldes AB, Alonso JL, Parajó JC (2003) Production of D (−)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 25:1161–1164
Benthin S, Villadsen J (1995) Production of optically pure D-lactate by Lactobacillus bulgaricus and purification by crystallisation and liquid/liquid extraction. Appl Microbiol Biotechnol 42:826–829
Okano K, Zhang Q, Shinkawa S et al (2009) Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl Environ Microbiol 75:462–467
Okano K, Zhang Q, Yoshida S et al (2010) D-lactic acid production from cellooligosaccharides and β-glucan using L-LDH gene-deficient and endoglucanase-secreting Lactobacillus plantarum. Appl Microbiol Biotechnol 85:643–650
Kleerebezem M, Hugenholtz J (2003) Metabolic pathway engineering in lactic acid bacteria. Curr Opin Biotechnol 14:232–237
Son J, Jeong KJ (2020) Recent advances in synthetic biology for the engineering of lactic acid bacteria. Biotechnol Bioprocess Eng 25:962–973
de Vos WM (1999) Gene expression systems for lactic acid bacteria. Curr Opin Microbiol 2:289–295
Cho SW, Yim J, Seo SW (2020) Engineering tools for the development of recombinant lactic acid bacteria. Biotechnol J 15:1900344
Rud I, Jensen PR, Naterstad K, Axelsson L (2006) A synthetic promoter library for constitutive gene expression in Lactobacillus plantarum. Microbiology (N Y) 152:1011–1019
Thompson J (1987) Regulation of sugar transport and metabolism in lactic acid bacteria. FEMS Microbiol Rev 3:221–231
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 49:209–224
Bhowmik T, Steele JL (1994) Cloning, characterization and insertional inactivation of the Lactobacillus helveticus D (−) lactate dehydrogenase gene. Appl Microbiol Biotechnol 41:432–439
Okano K, Tanaka T, Ogino C et al (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423
Saitoh S, Ishida N, Onishi T et al (2005) Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl Environ Microbiol 71:2789–2792
Okano K, Uematsu G, Hama S et al (2018) Metabolic engineering of Lactobacillus plantarum for direct l-lactic acid production from raw corn starch. Biotechnol J 13:1700517
Andersen HW, Solem C, Hammer K, Jensen PR (2001) Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux. J Bacteriol 183:3458–3467
de Felipe FL, Hugenholtz J (2001) Purification and characterisation of the water forming NADH-oxidase from Lactococcus lactis. Int Dairy J 11:37–44
Zhai Z, An H, Wang G et al (2015) Functional role of pyruvate kinase from Lactobacillus bulgaricus in acid tolerance and identification of its transcription factor by bacterial one-hybrid. Sci Rep 5:17024. https://doi.org/10.1038/srep17024
Awasthi D, Wang L, Rhee MS et al (2018) Metabolic engineering of Bacillus subtilis for production of D-lactic acid. Biotechnol Bioeng 115:453–463. https://doi.org/10.1002/BIT.26472
Ishida N, Suzuki T, Tokuhiro K et al (2006) d-Lactic acid production by metabolically engineered Saccharomyces cerevisiae. J Biosci Bioeng 101:172–177. https://doi.org/10.1263/JBB.101.172
Grabar TB, Zhou S, Shanmugam KT et al (2006) Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(-)-lactate fermentations by recombinant Escherichia coli. Biotechnol Lett 28:1527–1535. https://doi.org/10.1007/S10529-006-9122-7/METRICS
Wang Q, Ingram LO, Shanmugam KT (2011) Evolution of D-lactate dehydrogenase activity from glycerol dehydrogenase and its utility for D-lactate production from lignocellulose. Proc Natl Acad Sci U S A 108:18920–18925. https://doi.org/10.1073/PNAS.1111085108/SUPPL_FILE/PNAS.1111085108_SI.PDF
Baek SH, Kwon EY, Kim YH, Hahn JS (2016) Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 100:2737–2748. https://doi.org/10.1007/S00253-015-7174-0/TABLES/2
Sangproo M, Polyiam P, Jantama SS et al (2012) Metabolic engineering of Klebsiella oxytoca M5a1 to produce optically pure d-lactate in mineral salts medium. Bioresour Technol 119:191–198. https://doi.org/10.1016/J.BIORTECH.2012.05.114
In S, Khunnonkwao P, Wong N et al (2020) Combining metabolic engineering and evolutionary adaptation in Klebsiella oxytoca KMS004 to significantly improve optically pure D-(−)-lactic acid yield and specific productivity in low nutrient medium. Appl Microbiol Biotechnol 104:9565–9579. https://doi.org/10.1007/S00253-020-10933-0/TABLES/3
Zhang Y, Vadlani PV, Kumar A et al (2016) Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl Microbiol Biotechnol 100:279–288. https://doi.org/10.1007/S00253-015-7016-0/FIGURES/3
Tsuge Y, Kato N, Yamamoto S et al (2019) Metabolic engineering of Corynebacterium glutamicum for hyperproduction of polymer-grade l- and d-lactic acid. Appl Microbiol Biotechnol 103:3381–3391. https://doi.org/10.1007/S00253-019-09737-8/TABLES/3
Yamada R, Wakita K, Mitsui R, Ogino H (2017) Enhanced d-lactic acid production by recombinant Saccharomyces cerevisiae following optimization of the global metabolic pathway. Biotechnol Bioeng 114:2075–2084. https://doi.org/10.1002/BIT.26330
Zhong W, Yang M, Hao X et al (2021) Improvement of D-lactic acid production at low pH through expressing acid-resistant gene IoGAS1 in engineered Saccharomyces cerevisiae. J Chem Technol Biotechnol 96:732–742. https://doi.org/10.1002/JCTB.6587
Varman AM, Yu Y, You L, Tang YJ (2013) Photoautotrophic production of D-lactic acid in an engineered cyanobacterium. Microb Cell Fact 12:1–8. https://doi.org/10.1186/1475-2859-12-117/TABLES/2
Watcharawipas A, Sae-Tang K, Sansatchanon K et al (2021) Systematic engineering of Saccharomyces cerevisiae for D-lactic acid production with near theoretical yield. FEMS Yeast Res 21. https://doi.org/10.1093/FEMSYR/FOAB024
Heiss S, Hörmann A, Tauer C et al (2016) Evaluation of novel inducible promoter/repressor systems for recombinant protein expression in Lactobacillus plantarum. Microb Cell Fact 15:1–17
Van Pijkeren J-P, Britton RA (2012) High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res 40:e76–e76
Van Dien S (2013) From the first drop to the first truckload: commercialization of microbial processes for renewable chemicals. Curr Opin Biotechnol 24:1061–1068
Availability of data and materials
All the data and materials generated in this research work have been included in this study.
Funding
The work was supported by the Department of Biotechnology (Ministry of Science and Technology, Govt. of India) via project no. BT/PR24592/NER/95/764/2017.
Author information
Authors and Affiliations
Contributions
PM: conceptualization, formal analysis, validation, investigation, writing – original draft, writing—review and editing; NR: investigation, diagrams, and tables—review and editing; SS: supervision, project administration, funding acquisition, writing—review, and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukherjee, P., Raj, N. & Sivaprakasam, S. Harnessing valorization potential of whey permeate for D-lactic acid production using lactic acid bacteria. Biomass Conv. Bioref. 13, 15639–15658 (2023). https://doi.org/10.1007/s13399-023-05038-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-05038-3