Abstract
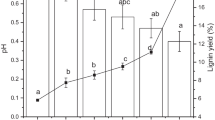
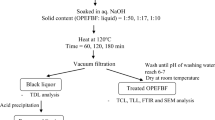
Lignin has potency as a seed coating treatment for preserving seed from deterioration prior to plant and prevent seed growth quickly. Nonetheless, given the complexity of lignin characteristics, it is necessary to develop isolation methods to ensure appropriate utilization, particularly for seed coating. This study intended to observe the oil palm empty fruit bunch (OPEFB) lignin for seed coating treatment and evaluate the effect on chili seed growth. Previously, lignin was extracted by inorganic acid, specifically H2SO4 and HCL, via a precipitation method with washing treatment of 3 and 5 times. The effectiveness of lignin as seed coating was evaluated by soaking chili seeds in a lignin-NaOH solution. The results showed that HCl-isolated lignin has a higher purity of 91.62% compared to H2SO4-isolated lignin after 5 washing treatments. Meanwhile, H2SO4-isolated lignin has a higher yield of 19.29%. The syringyl/guiacyl (S/G) ratio of isolated lignin by HCl and H2SO4 with different washing treatments ranges from 0.97 to 1.97. Thermal analysis reveals that HCl-isolated lignin is more stable than H2SO4-isolated lignin, with a mass residue of 49.4%. The glass transition (Tg) ranges from 126.42 to 168.05 °C. The result showed that the shelf life of lignin-coated seed was up to 30 weeks at room temperature and more resistance to fungal contaminants. Germination of chili-coated seeds occurs in the second to fourth weeks after the planting period.




Similar content being viewed by others
Data availability
Not applicable.
References
Statista (2022) Production volume of palm oil in Indonesia from 2012 to 2021. In: Stat Res Dep. https://www.statista.com/statistics/706786/production-of-palm-oil-in-indonesia/. Accessed 4 Apr 2023
IPOA (2023) Palm oil industry performance February 2023: production decline still continues. In: GAPKI Indones. Palm Oil Assoc. https://gapki.id/en/news/23012/palm-oil-industry-performance-february-2023-production-decline-still-continues. Accessed 14 Aug 2023
IPOA (2022) Palm oil performance in 2021 and prospect in 2022. In: GAPKI Indones. Palm Oil Assoc. https://gapki.id/en/news/21136/palm-oil-performance-in-2021-and-prospect-in-2022. Accessed 14 Apr 2023
Mahidin, Saifullah, Erdiwansyah et al (2020) Analysis of power from palm oil solid waste for biomass power plants: A case study in Aceh Province. Chemosphere 253:126714. https://doi.org/10.1016/j.chemosphere.2020.126714
Li YH, Chen HH (2018) Analysis of syngas production rate in empty fruit bunch steam gasification with varying control factors. Int J Hydrog Energy 43:667–675. https://doi.org/10.1016/j.ijhydene.2017.11.117
Sari FP, Falah F, Anita SH et al (2021) Pretreatment of oil palm empty fruit bunch (OPEFB) at bench-scale high temperature-pressure steam reactor for enhancement of enzymatic saccharification. Int J Renew Energy Dev 10:157–169. https://doi.org/10.14710/ijred.2021.32343
Dewanti DP (2018) Potensi Selulosa dari Limbah Tandan Kosong Kelapa Sawit untuk Bahan Baku Bioplastik Ramah Lingkungan. J Teknol Lingkung 19:81. https://doi.org/10.29122/jtl.v19i1.2644
Ridho MR, Agustiany EA, Rahmi Dn M et al (2022) Lignin as green filler in polymer composites: development methods, characteristics, and potential applications. Adv Mater Sci Eng 10:1–33. https://doi.org/10.1155/2022/1363481
Ela RCA, Spahn L, Safaie N et al (2020) Understanding the effect of precipitation process variables on hardwood lignin characteristics and recovery from black liquor. ACS Sustain Chem Eng 8:13997–14005. https://doi.org/10.1021/acssuschemeng.0c03692
Chen J, Fan X, Zhang L et al (2020) Research progress in lignin-based slow/controlled release fertilizer. Chem Sustain Energy Mater 13:4356–4366. https://doi.org/10.1002/cssc.202000455
Surina I, Jablonsky M, Haz A et al (2015) Characterisation of non-wood lignin precipitated with sulphuric acid of various concentration. BioResources 10:1408–1423
Xu T, Ma C, Aytac Z et al (2020) Enhancing agrichemical delivery and seedling development with biodegradable, tunable, biopolymer-based nanofiber seed coatings. ACS Sustain Chem Eng 8:9537–9548. https://doi.org/10.1021/acssuschemeng.0c02696
Kienberger M, Maitz S, Pichler T, Demmelmayer P (2021) Systematic review on isolation processes for technical lignin. Processes 9. https://doi.org/10.3390/pr9050804
Lourençon TV, de Lima GG, Ribeiro CSP et al (2021) Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int J Biol Macromol 166:1535–1542. https://doi.org/10.1016/j.ijbiomac.2020.11.033
Poursorkhabi V, Misra M, Mohanty AK (2013) Extraction of lignin from a coproduct of the cellulosic ethanol industry and its thermal characterization. BioResources 8:5083–5101. https://doi.org/10.15376/biores.8.4.5083-5101
Yang Q, Pan X (2016) Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol Bioeng 113:1213–1224. https://doi.org/10.1002/bit.25903
Yang J, Ching YC, Chuah CH (2019) Applications of lignocellulosic fibers and lignin in bioplastics: a review. Polymers 11:1–26
Zikeli F, Vettraino AM, Biscontri M et al (2023) Lignin nanoparticles with entrapped Thymus spp. essential oils for the control of wood-rot fungi. Polymers (Basel) 15:2713. https://doi.org/10.3390/polym15122713
Deepa GT, Chetti MB, Khetagoudar MC, Adavirao GM (2013) Influence of vacuum packaging on seed quality and mineral contents in chilli (Capsicum annuum L.). J Food Sci Technol 50:153–158. https://doi.org/10.1007/s13197-011-0241-3
Sukowardojo B, Pertanian F, Jember U (2012) Upaya memperpanjang daya simpan benih kedelai dengan pelapisan chitosan berdasarkan penilaian viabilitas dan kandungan kimiawi. J Agritrop 11:15–22. https://doi.org/10.32528/agr.v11i1.664
Afzal I, Javed T, Amirkhani M, Taylor AG (2020) Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agric 10:1–20. https://doi.org/10.3390/agriculture10110526
Kimmelshue C, Goggi AS, Cademartiri R (2019) The use of biological seed coatings based on bacteriophages and polymers against Clavibacter michiganensis subsp. nebraskensis in maize seeds. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-54068-3
Gorim L, Asch F (2012) Effects of composition and share of seed coatings on the mobilization efficiency of cereal seeds during germination. J Agron Crop Sci 198:81–91. https://doi.org/10.1111/j.1439-037X.2011.00490.x
Amirkhani M, Mayton HS, Netravali AN, Taylor AG (2019) A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustain 11:1–16. https://doi.org/10.3390/su11195304
Solihat NN, Santoso EB, Karimah A et al (2022) Physical and chemical properties of Acacia mangium lignin isolated from pulp mill byproduct for potential application in wood composites. Polymers (Basel) 14:1–19. https://doi.org/10.3390/polym14030491
TAPPI Test Method T 264 cm-97 (1997) Preparation of wood for chemical analysis. In: Technical Association of the Pulp & Paper Industry. Peachtree Corners, USA, pp 3–5
TAPPI T211 om-02 method (2007) Ash in wood, pulp, paper and paperboard: Combustion at 525 °C. Peachtree Corners, USA
Sluiter A, Hames B, Ruiz R et al (2012) Determination of structural carbohydrates and lignin in Biomass - NREL/TP-510-42618. [NREL] National Renewable Energy Laboratory, pp 1–17
Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 12. https://doi.org/10.1186/1472-6882-12-221
Serrano L, Esakkimuthu ES, Marlin N et al (2018) Fast, easy, and economical quantification of lignin phenolic hydroxyl groups: comparison with classical techniques. Energy Fuel 32:5969–5977. https://doi.org/10.1021/acs.energyfuels.8b00383
Nakagawa-Izumi A, H’ng YY, Mulyantara LT et al (2017) Characterization of syringyl and guaiacyl lignins in thermomechanical pulp from oil palm empty fruit bunch by pyrolysis-gas chromatography-mass spectrometry using ion intensity calibration. Ind Crop Prod 95:615–620. https://doi.org/10.1016/j.indcrop.2016.11.030
Surono NK (2017) The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus of fi cinalis growth. Fungal Ecol 28:1–10. https://doi.org/10.1016/j.funeco.2017.04.001
Ridho MR, Madyaratri EW, Agustiany EA et al (2022) Isolation and characterization of lignin from black liquor of arecanut leaf sheath (Areca catechu L.) with hydrochloric acid and phosphoric acid. Int Conf Lignocellul:1–9
Hermiati E, Risanto L, Lubis MAR et al (2017) Chemical characterization of lignin from kraft pul** black liquor of Acacia mangium. AIP Conf Proc 1803. https://doi.org/10.1063/1.4973132
Vishtal A, Kraslawski A (2011) Challenges in industrial applications of technical lignins. BioResources 6:3547–3568. https://doi.org/10.15376/biores.6.3.vishtal
Risanto L, Hermiati E, Sudiyani Y (2014) Properties of lignin from oil palm empty fruit bunch and its application for plywood adhesive. Makara J Technol 18:67. https://doi.org/10.7454/mst.v18i2.2944
Lubis MAR, Dewi AR, Risanto L et al (2014) Isolation and characterization of lignin from alkaline pretreatment black liquor of oil palm empty fruit bunch and sugarcane bagasse. Proceeding Asean Cosat 2014:483–491
Ajao O, Jeaidi J, Benali M et al (2018) Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications. Molecules 23. https://doi.org/10.3390/molecules23020377
Mohtar SS, Tengku Malim Busu TNZ, Md Noor AM et al (2015) Extraction and characterization of lignin from oil palm biomass via ionic liquid dissolution and non-toxic aluminium potassium sulfate dodecahydrate precipitation processes. Bioresour Technol 192:212–218. https://doi.org/10.1016/j.biortech.2015.05.029
Ludmila H, Michal J, Andrea Š, Aleš H (2015) Lignin, potential products and their market value. Wood Res 60:973–986
Amran UA, Zakaria S, Chia CH et al (2017) Production of liquefied oil palm empty fruit bunch based polyols via microwave heating. Energy Fuel 31:1–34. https://doi.org/10.1021/acs.energyfuels.7b02098
Madyaratri EW, Iswanto AH, Nawawi DS et al (2022) Improvement of thermal behavior of rattan by lignosulphonate impregnation treatment. Forests 13:1–24. https://doi.org/10.3390/f13111773
Jung W, Savithri D, Sharma-Shivappa R, Kolar P (2020) Effect of sodium hydroxide pretreatment on lignin monomeric components of miscanthus × giganteus and enzymatic hydrolysis. Waste Biomass Valorization 11:5891–5900. https://doi.org/10.1007/s12649-019-00859-8
Ohra-Aho T, Gomes FJB, Colodette JL, Tamminen T (2013) S/G ratio and lignin structure among Eucalyptus hybrids determined by Py-GC/MS and nitrobenzene oxidation. J Anal Appl Pyrolysis 101:166–171. https://doi.org/10.1016/j.jaap.2013.01.015
Zhang Y, Naebe M (2021) Lignin: a review on structure, properties, and applications as a light-colored UV absorber. ACS Sustain Chem Eng 9:1427–1442. https://doi.org/10.1021/acssuschemeng.0c06998
Yu O, Kim KH (2020) Lignin to materials: A focused review on recent novel lignin applications. Appl Sci 10. https://doi.org/10.3390/app10134626
Coral Medina JD, Woiciechowski A, Zandona Filho A et al (2015) Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment — a biorefinery approach. Bioresour Technol 194:172–178. https://doi.org/10.1016/j.biortech.2015.07.018
Díez D, Urueña A, Piñero R et al (2020) Determination of hemicellulose, cellulose, and lignin content in different types of biomasses by thermogravimetric analysis and pseudocomponent kinetic model (TGA-PKM Method). Processes 8. https://doi.org/10.3390/pr8091048
Manara P, Zabaniotou A, Vanderghem C, Richel A (2014) Lignin extraction from Mediterranean agro-wastes: Impact of pretreatment conditions on lignin chemical structure and thermal degradation behavior. Catal Today 223:25–34. https://doi.org/10.1016/j.cattod.2013.10.065
Handika SO, Lubis MAR, Sari RK et al (2021) Enhancing thermal and mechanical properties of ramie fiber via impregnation by lignin-based polyurethane resin. Materials (Basel) 14:1–22. https://doi.org/10.3390/ma14226850
El MM, El KA, El MM et al (2018) Thermal and thermomechanical analyses of lignin. Sustain Chem Pharm 9:63–68. https://doi.org/10.1016/j.scp.2018.06.002
Aleš H, Michal J, Lenka D et al (2015) Thermal properties and size distribution of lignins precipitated with sulphuric acid. Wood Res 60:375–384
Dominguez-Robles J, Espinosa E, Savy D et al (2016) Biorefinery process combining specel process and selective lignin precipitation using mineral acid. Chem Eng 120:14
Sameni J, Krigstin S, Rosa D d S et al (2014) Thermal characteristics of lignin residue from industrial processes. BioResources 9:725–737. https://doi.org/10.15376/biores.9.1.725-737
Yue X, Suopajärvi T, Mankinen O et al (2020) Comparison of lignin fractions isolated from wheat straw using alkaline and acidic deep eutectic solvents. J Agric Food Chem 68:15074–15084. https://doi.org/10.1021/acs.jafc.0c04981
Maniet G, Schmetz Q, Jacquet N et al (2017) Effect of steam explosion treatment on chemical composition and characteristic of organosolv fescue lignin. Ind Crop Prod 99:79–85. https://doi.org/10.1016/j.indcrop.2017.01.015
Yun J, Wei L, Li W et al (2021) Isolating high antimicrobial ability lignin from bamboo kraft lignin by organosolv fractionation. Front Bioeng Biotechnol 9:1–11. https://doi.org/10.3389/fbioe.2021.683796
Bormashenko E, Grynyov R, Bormashenko Y, Drori E (2012) Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci Rep 2:3–10. https://doi.org/10.1038/srep00741
Jouki M, Khazaei N, Ghasemlou M, Hadinezhad M (2013) Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr Polym 96:39–46. https://doi.org/10.1016/j.carbpol.2013.03.077
Ben Sghaier AEO, Chaabouni Y, Msahli S, Sakli F (2012) Morphological and crystalline characterization of NaOH and NaOCl treated Agave americana L. fiber. Ind Crop Prod 36:257–266. https://doi.org/10.1016/j.indcrop.2011.09.012
Kok ADX, Muhamad W, Nizam A et al (2021) Sodium lignosulfonate improves shoot growth of Oryza sativa via enhancement of photosynthetic activity and reduced accumulation of reactive oxygen species. Sci Rep:1–13. https://doi.org/10.1038/s41598-021-92401-x
Haghighi M, Teixeira JA, Teixeira JA (2014) The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J Crop Sci Biotechnol 2014:201–208
Wang S, Su S, **ao LP et al (2020) Catechyl lignin extracted from castor seed coats using deep eutectic solvents: characterization and depolymerization. ACS Sustain Chem Eng 8:7031–7038. https://doi.org/10.1021/acssuschemeng.0c00462
ur Rehman H, Iqbal Q, Farooq M et al (2013) Sulphur application improves the growth, seed yield and oil quality of canola. Acta Physiol Plant 35:2999–3006. https://doi.org/10.1007/s11738-013-1331-9
Schmitt M, Watanabe T, Jansen S (2016) The effects of aluminium on plant growth in a temperate and deciduous aluminium accumulating species. AoB Plants 8. https://doi.org/10.1093/aobpla/plw065
Ahmed AKA, Shi X, Hua L et al (2018) Influences of air, oxygen, nitrogen, and carbon dioxide nanobubbles on seed germination and plant growth. J Agric Food Chem 66:5117–5124. https://doi.org/10.1021/acs.jafc.8b00333
Ahmed N, Masood A, Siow KS et al (2022) Effects of oxygen (O2) plasma treatment in promoting the germination and growth of chili. Plasma Chem Plasma Process 42:91–108. https://doi.org/10.1007/s11090-021-10206-2
Ceccarelli N, Curadi M, Picciarelli P et al (2010) Globe artichoke as a functional food. Med J Nutrition Metab 3:197–201. https://doi.org/10.1007/s12349-010-0021-z
Rouphael Y, Colla G, Graziani G et al (2017) Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem 234:10–19. https://doi.org/10.1016/j.foodchem.2017.04.175
Acknowledgements
The authors acknowledge the Advanced Characterization Laboratories Cibinong—Integrated Laboratory of Bioproduct (i-Lab), National Research and Innovation Agency, for the facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization: Widya Fatriasari, Ikhsan Guswenrivo, and Deded Sarip Nawawi; methodology: Widya Fatriasari and Erika Ayu Agustiany; software: Erika Ayu Agustiany and Krisna Suzana; validation: Widya Fatriasari and Deded Sarip Nawawi; formal analysis: Widya Fatriasari, Ikhsan Guswenrivo, and Deded Sarip Nawawi; investigation: Widya Fatriasari; resources: Widya Fatriasari and Ikhsan Guswenrivo; data curation: Erika Ayu Agustiany, Ikhsan Guswenrivo, and Widya Fatriasari; writing—original draft preparation: Erika Ayu Agustiany, Ikhsan Guswenrivo, Widya Fatriasari, Deded Sarip Nawawi, and Arief Heru Priyanto; writing—review and editing: Widya Fatriasari, Ikhsan Guswenrivo, and Deded Sarip Nawawi; visualization: Erika Ayu Agustiany and Krisna Suzana; supervision: Widya Fatriasari and Deded Sarip Nawawi; project administration: Widya Fatriasari; funding acquisition: Widya Fatriasari. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agustiany, E.A., Suzana, K., Guswenrivo, I. et al. The effect of the lignin isolation method from oil palm empty fruit bunch black liquor for seed coating material. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04874-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04874-7