Abstract
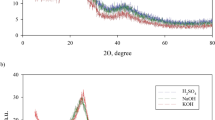
Activated carbon (ACcs) from chili straw was prepared by sequential carbonization and K2CO3 activation and applied for adsorption of cationic (methylene blue (MB)) and anionic (methyl orange (MO)) dyes with similar molecular sizes from aqueous solution. The physicochemical properties of chili straw char (Charcs) and ACcs were compared based on N2-adsorption/desorption, SEM, XRD, Raman spectroscopy, and FTIR. Activation mechanism was revealed with the aid of TG-FTIR. Batch experiments were performed to determine the isotherm models for adsorption of dyes. Results showed that ACcs possessed a large specific surface area of 1868 m2 g−1. The formation of pores was attributed to the reactions between oxygen-containing functional groups in Charcs and K2CO3 at temperatures of 670 to 850 °C. Sips model was applicable for the adsorption isotherm data for both MO and MB with outstanding maximum adsorption capacities of 1542 and 989 mg g−1, respectively. Thermodynamic analysis revealed that the adsorption processes of MO and MB were endothermic and spontaneous, and the adsorption was more spontaneous in the case of MO. Zeta potential analysis revealed that electrostatic interactions were responsible for the significant difference between the adsorption capacities for MO and MB. Thermal desorption MO from ACcs was investigated by TG-FTIR, and thermal regeneration of MO-saturated ACcs was carried out at 650 °C for 30 min, and the regeneration efficiency remained higher than 60% after five cycles.

















Similar content being viewed by others
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Alam S, Khan MS, Bibi W, Zekker I, Burlakovs J, Ghangrekar MM et al (2021) Preparation of activated carbon from the wood of Paulownia tomentosa as an efficient adsorbent for the removal of acid red 4 and methylene blue present in wastewater. Water 13(11):1453
Wang Q, Luo C, Lai Z, Chen S, He D, Mu J (2022) Honeycomb-like cork activated carbon with ultra-high adsorption capacity for anionic, cationic and mixed dye: preparation, performance and mechanism. Bioresource Technol 357:127363
Lin J, Zhao S, Cheng S (2022) Microwave-assisted preparation of cotton stem-derived activated carbon for dye removal from synthetic wastewater. Environ Sci Pollut R 29(32):48839–48850
Chan AA, Buthiyappan A, Raman AAA, Ibrahim S (2022) Recent advances on the coconut shell derived carbonaceous material for the removal of recalcitrant pollutants: a review. Korean J Chem Eng 39(10):2571–2593
Ben Moshe S, Furman A (2022) Real-time monitoring of organic contaminant adsorption in activated carbon filters using spectral induced polarization. Water Res 212:118103
Ahmad AA, Al-Raggad M, Shareef N (2021) Production of activated carbon derived from agricultural by-products via microwave-induced chemical activation: a review. Carbon Lett 31(5):957–971
Feng J, Zhu H, Xu Y, Jiang J, Pan H (2021) Preparation and characterization of high-performance activated carbon from papermaking black-liquor at low temperature. J Anal Appl Pyrol 159:105292
Samiyammal P, Kokila A, Pragasan LA, Rajagopal R, Sathya R, Ragupathy S et al (2022) Adsorption of brilliant green dye onto activated carbon prepared from cashew nut shell by KOH activation: studies on equilibrium isotherm. Environ Res 212:113497
Ma X, Wu Y, Fang M, Liu B, Chen R, Shi R et al (2022) In-situ activated ultramicroporous carbon materials derived from waste biomass for CO2 capture and benzene adsorption. Biomass Bioenerg 158:106353
Magesh N, Renita AA, Siva R, Harirajan N, Santhosh A (2022) Adsorption behavior of fluoroquinolone (ciprofloxacin) using zinc oxide impregnated activated carbon prepared from jack fruit peel: Kinetics and isotherm studies. Chemosphere 290:133227
Wei M, Marrakchi F, Yuan C, Cheng X, Jiang D, Zafar FF et al (2022) Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J Hazard Mater 425:127887
Yang Y, Cannon FS (2022) Biomass activated carbon derived from pine sawdust with steam bursting pretreatment; perfluorooctanoic acid and methylene blue adsorption. Bioresour Technol 344:126161
Sharma G, Sharma S, Kumar A, Lai CW, Naushad M, Shehnaz et al (2022) Activated carbon as superadsorbent and sustainable material for diverse applications. Adsorpt Sci Technol 2022:1–21
Zhang X, Yu Z, Lu X, Ma X (2021) Catalytic co-pyrolysis of microwave pretreated chili straw and polypropylene to produce hydrocarbons-rich bio-oil. Bioresour Technol 319:124191
Yu F, Wang Y, **e Y, Zhang W, Zhang J, Meng X et al (2019) A Microtubular Direct Carbon Solid Oxide Fuel Cell Operated on the Biochar Derived from Pepper Straw. Energ Technol 8(3):1901077
Hu L, Wei X-Y, Guo X-H, Lv H-P, Wang G-H (2021) Investigation on the kinetic behavior, thermodynamic and volatile products analysis of chili straw waste pyrolysis. J Environ Chem Eng 9(5):105859
Zhang X, Ma X, Yu Z, Yi Y, Huang Z, Lu C (2022) Preparation of high-value porous carbon by microwave treatment of chili straw pyrolysis residue. Bioresource Technol 360:127520
Shou J, Qiu M (2016) Adsorption of copper ions onto activated carbon from capsicum straw. Desalin Water Treat 57:353–359
Sevilla M, Diez N, Fuertes AB (2021) More sustainable chemical activation strategies for the production of porous carbons. Chemsuschem 14(1):94–117
Zhang S, Tao L, Jiang M, Gou G, Zhou Z (2015) Single-step synthesis of magnetic activated carbon from peanut shell. Mater Lett 157:281–284
Adinata D, Wan Daud WM, Aroua MK (2007) Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresource Technol 98(1):145–149
Gil RR, Ruiz B, Lozano MS, Martín MJ, Fuente E (2014) VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem Eng J 245:80–88
Liu X, Tian J, Li Y, Sun N, Mi S, **e Y et al (2019) Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J Hazard Mater 373:397–407
Chen H, Ferrari C, Angiuli M, Yao J, Raspi C, Bramanti E (2010) Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohyd Polym 82(3):772–778
Tserki V, Matzinos P, Kokkou S, Panayiotou C (2005) Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I. Surface chemical modification and characterization of waste flour. Compos Part A: Appl S 36(7):965–974
Chen D, Cen K, Zhuang X, Gan Z, Zhou J, Zhang Y et al (2022) Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust Flame 242:112142
Zhang B, Yang D, Qiu X, Qian Y, Yan M, Li Q (2020) Influences of aggregation behavior of lignin on the microstructure and adsorptive properties of lignin-derived porous carbons by potassium compound activation. J Ind Eng Chem 82:220–227
Rosson E, Sgarbossa P, Mozzon M, Venturino F, Bogialli S, Glisenti A et al (2021) Novel correlations between spectroscopic and morphological properties of activated carbons from waste coffee grounds. Processes 9(9):1637
Gao P, Zhang Y, Du J, Sui H, He L (2021) Preparation and application of porous activated carbon using phenolic distillation residue. J Mater Sci 56(30):16902–16915
Yu H, Son YR, Yoo H, Cha HG, Lee H, Kim HS (2019) Chitosan-derived porous activated carbon for the removal of the chemical warfare agent simulant dimethyl methylphosphonate. Nanomaterials 9:1703
Zhang Y, Kang X, Tan J, Frost RL (2013) Influence of calcination and acidification on structural characterization of Anyang anthracites. Energ Fuel 27(11):7191–7197
Zhong M, Gao S, Zhou Q, Yue J, Ma F, Xu G (2016) Characterization of char from high temperature fluidized bed coal pyrolysis in complex atmospheres. Particuology 25:59–67
Nasser R, Tiantian J, Song J-M (2022) Hierarchical porous activated carbon derived from olives: preparation, (N, S) co-do**, and its application in supercapacitors. J Energy Storage 51:104348
Croce A, Re G, Bisio C, Gatti G, Coluccia S, Marchese L (2021) On the correlation between Raman spectra and structural properties of activated carbons derived by hyper-crosslinked polymers. Res Chem Intermediat 47(1):419–431
Fakhar N, Ayoub Khan S, Ahmad Siddiqi W, Alam Khan T (2021) Ziziphus jujube waste-derived biomass as cost-effective adsorbent for the sequestration of Cd2+ from aqueous solution: isotherm and kinetics studies. Environ Nanotechnol Monit Manage 16:100570
Liu P, Wang L, Zhou Y, Pan T, Lu X, Zhang D (2016) Effect of hydrothermal treatment on the structure and pyrolysis product distribution of **aolongtan lignite. Fuel 164:110–118
Gheitasi F, Ghammamy S, Zendehdel M, Semiromi FB (2022) Removal of mercury (II) from aqueous solution by powdered activated carbon nanoparticles prepared from beer barley husk modified with Thiol/Fe3O4. J Mol Struct 1267:133555
Wang K, Zhao N, Lei S, Yan R, Tian X, Wang J et al (2015) Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim Acta 166:1–11
Luo J, Lu J, Niu Q, Chen X, Wang Z, Zhang J (2015) Preparation and characterization of benzoic acid-modified activated carbon for removal of gaseous mercury chloride. Fuel 160:440–445
Chia CH, Gong B, Joseph SD, Marjo CE, Munroe P, Rich AM (2012) Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib Spectrosc 62:248–257
Mechati F, Bouchelta C, Medjram MS, Benrabaa R, Ammouchi N (2015) Effect of hard and soft structure of different biomasses on the porosity development of activated carbon prepared under N2/microwave radiations. J Environ Chem Eng 3(3):1928–1938
Kharel G, Sacko O, Feng X, Morris JR, Phillips CL, Trippe K et al (2019) Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain Chem Eng 7(19):16410–16418
Yin L, Leng E, Fang Y, Liu T, Gong X, Zhou J (2020) Effects of KCl, KOH and K2CO3 on the pyrolysis of Cβ-O type lignin-related polymers. J Anal Appl Pyrol 147:104809
Zhou Y, Li Z, Ji L, Wang Z, Cai L, Guo J et al (2022) Facile preparation of alveolate biochar derived from seaweed biomass with potential removal performance for cationic dye. J Mol Liq 353:118623
Liu J, Yang X, Liu H, Jia X, Bao Y (2021) Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: co-pyrolysis characteristics and its adsorption capability. Chemosphere 282:131116
Long Y, Ruan L, Lv X, Lv Y, Su J, Wen Y (2015) TG–FTIR analysis of pyrolusite reduction by major biomass components. Chinese J Chem Eng 23(10):1691–1697
Lehman RL, Gentry JS, Glumac NG (1998) Thermal stability of potassium carbonate near its melting point. Thermochim Acta 316:1–9
Hwang JY, Yu JH, Kang K (2015) A study of the gasification of carbon black with molten salt of Li2CO3 and K2CO3 for application in the external anode media of a direct carbon fuel cell. Curr Appl Phys 15(12):1580–1586
Kim Y-K, Hao L-f, Park J-I, Miyawaki J, Mochida I, Yoon S-H (2012) Catalytic activity and activation mechanism of potassium carbonate supported on perovskite oxide for coal char combustion. Fuel 94:516–522
Lu C, Xu S, Liu C (2010) The role of K2CO3 during the chemical activation of petroleum coke with KOH. J Anal Appl Pyrol 87(2):282–287
Zhu H, Wang X, Wang F, Yu G (2018) In situ study on K2CO3-catalyzed CO2 gasification of coal char: interactions and char structure evolution. Energ Fuel 32(2):1320–1327
Jiang L, Hu S, Xu K, Wang Y, Syed-Hassan SSA, Su S et al (2017) Formation, fates and roles of catalytic precursors generated from the K2CO3-carbon interactions in the K2CO3-catalyzed CO2 gasification of coal char. J Anal Appl Pyrol 124:384–392
Gao Y, Zhang Y, Ma Y (2022) Bio-inspired hierarchical porous activated carbon aerogel from waste corrugated cardboard for adsorption of oxytetracycline from water. Biomass Convers Bior. https://doi.org/10.1007/s13399-022-02936-w
Mahmoudi K, Hosni K, Hamdi N, Srasra E (2014) Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits-A comparative study. Korean J Chem Eng 32(2):274–283
Gong R, Ye J, Dai W, Yan X, Hu J, Hu X et al (2013) Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind Eng Chem Res 52(39):14297–14303
Tao X, Wu Y, Cha L (2019) Shaddock peels-based activated carbon as cost-saving adsorbents for efficient removal of Cr (VI) and methyl orange. Environ Sci Polluti R 26(19):19828–19842
Jiang X, **ang X, Peng S, Hou L (2019) Facile preparation of nitrogen-doped activated mesoporous carbon aerogel from chitosan for methyl orange adsorption from aqueous solution. Cellulose 26(7):4515–4527
Mahmoudi K, Hamdi N, Kriaa A, Srasra E (2012) Adsorption of methyl orange using activated carbon prepared from lignin by ZnCl2 treatment. Russ J Phys Chem 86(8):1294–1300
Azmi NH, Ali UFM, Muhammad Ridwan F, Isa KM, Zulkurnai NZ, Aroua MK (2016) Preparation of activated carbon using sea mango (Cerbera odollam) with microwave-assisted technique for the removal of methyl orange from textile wastewater. Desalin Water Treat 57(60):29143–29152
Chen S, Zhang J, Zhang C, Yue Q, Li Y, Li C (2010) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 252(1–3):149–156
Saini K, Sahoo A, Biswas B, Kumar A, Bhaskar T (2021) Preparation and characterization of lignin-derived hard templated carbon(s): statistical optimization and methyl orange adsorption isotherm studies. Bioresource Technol 342:125924
Hou XX, Deng QF, Ren TZ, Yuan ZY (2013) Adsorption of Cu2+ and methyl orange from aqueous solutions by activated carbons of corncob-derived char wastes. Environ Sci Polluti R 20(12):8521–8534
Mbarki F, Selmi T, Kesraoui A, Seffen M (2022) Low-cost activated carbon preparation from corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: Study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind Crop Prod 178:114546
El Malti W, Hijazi A, Khalil ZA, Yaghi Z, Medlej MK, Reda M (2022) Comparative study of the elimination of copper, cadmium, and methylene blue from water by adsorption on the citrus Sinensis peel and its activated carbon. RSC adv 12(17):10186–10197
Misran E, Bani O, Situmeang EM, Purba AS (2022) Banana stem based activated carbon as a low-cost adsorbent for methylene blue removal: isotherm, kinetics, and reusability. Alex Eng J 61(3):1946–1955
Zhao H, Zhong H, Jiang Y, Li H, Tang P, Li D et al (2022) Porous ZnCl2-activated carbon from shaddock peel: methylene blue adsorption behavior. Materials 15:895
Lawtae P, Tangsathitkulchai C (2021) The use of high surface area mesoporous-activated carbon from longan seed biomass for increasing capacity and kinetics of methylene blue adsorption from aqueous solution. Molecules 26:6521
Huang F, Bi H, Zhou F (2021) Ultra-large specific surface area activated carbon synthesized from rice husk with high adsorption capacity for methylene blue. J Inorg Mater 36(8):893
Zhang Z, Xu L, Liu Y, Feng R, Zou T, Zhang Y et al (2021) Efficient removal of methylene blue using the mesoporous activated carbon obtained from mangosteen peel wastes: kinetic, equilibrium, and thermodynamic studies. Micropor Mesopor Mater 315:110904
Zhou D, Li D, Li A, Qi M, Cui D, Wang H et al (2021) Activated carbons prepared via reflux-microwave-assisted activation approach with high adsorption capability for methylene blue. J Environ Chem Eng 9(1):104671
Bouzikri S, Ouasfi N, Khamliche L (2022) Bifurcaria bifurcata activated carbon for the adsorption enhancement of Acid Orange 7 and Basic Red 5 dyes: kinetics, equilibrium and thermodynamics investigations. Energy Nexus 7:100138
Zhuang X, Wan Y, Feng C, Shen Y, Zhao D (2009) Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem Mater 21:706–716
Liang T, Wang F, Liang L, Liu M, Sun J (2016) Magnetically separable nitrogen-doped mesoporous carbon with high adsorption capacity. J Mater Sci 51(8):3868–3879
Han Y, Sheng S, Yang F, **e Y, Zhao M, Li J-R (2015) Size-exclusive and coordination-induced selective dye adsorption in a nanotubular metal–organic framework. J Mater Chem A 3(24):12804–12809
Wang C-C, Li J-R, Lv X-L, Zhang Y-Q, Guo G (2014) Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ Sci 7(9):2831–2867
Hadi P, Yeung KY, Barford J, An KJ, McKay G (2015) Significance of “effective” surface area of activated carbons on elucidating the adsorption mechanism of large dye molecules. J Environ Chem Eng 3(2):1029–1037
Wang M, Day S, Wu Z, Wan X, Ye X, Cheng B (2021) A new type of porous Zn (II) metal-organic gel designed for effective adsorption to methyl orange dye. Colloid Surface A 628:127335
Funding
This work was financially supported by the National Natural Science Foundation of China (51909292), and the Fundamental Research Funds for Central Public Welfare Scientific Research Institution (K-JBYWF-2021-ZT04, R-JBYWF-2021-D05, R-JBYWF-2021-D04).
Author information
Authors and Affiliations
Contributions
Xunliang Wang: investigation, conceptualization, formal analysis, writing—original draft.
Xuemin Feng: investigation, formal analysis, writing—revised draft.
Yuhui Ma: funding acquisition, supervision, conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Feng, X. & Ma, Y. Activated carbon from chili straw: K2CO3 activation mechanism, adsorption of dyes, and thermal regeneration. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04173-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04173-1