Abstract
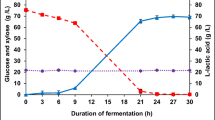
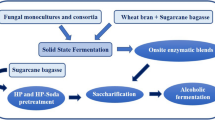
Bioemulsifier production by Aureobasidium pullulans LB83 from pretreated sugarcane bagasse was evaluated in solid-state fermentation. Alkaline (0.2 mol.L−1 NaOH; 12 min) and hydrodynamic cavitation (0.5 mol.L−1 NaOH, 25 min) pretreatments showed a maximum lignin removal of 55.1 % and 44.7 %, respectively. Pretreatment biomass effectivity was assessed by X-ray diffraction, FT-MIR, FTIR-NIR, and RAMAN techniques. Maximum kerosene emulsification indexes of 65.8 % and 41.3 % were obtained in solid-state fermentation, respectively, for alkaline and hydrodynamic cavitation pretreated sugarcane bagasse. Synthesis of cellulases was observed in fermentation, showing maximum values of endoglucanase and exoglucanase, respectively, of 2.25 U.g−1 and 1.39 U.g−1, for alkaline pretreated sugarcane bagasse, and 2.43 U.g−1 and 1.45 U.g−1, for hydrodynamic cavitation-pretreated sugarcane bagasse. The biomolecule was characterized as a mixture of mannitol and arabitol-type liamocin. Microorganism was able to produce bioemulsifier using sugarcane bagasse as support and carbon source in solid-state fermentation, thus showing the potential of this system to obtain value-added products from this biomass.




Similar content being viewed by others
References
Botshekan M, Moheb A, Vatankhah F, Karimi K, Shafiei M (2021) Energy saving alternatives for renewable ethanol production with the focus on separation/purification units: a techno-economic analysis. Energy 122363. https://doi.org/10.1016/j.energy.2021.122363
Sanford K, Chotani G, Danielson N, Zahn JA (2016) Scaling up of renewable chemicals. Curr Opin Biotechnol 38:112–122. https://doi.org/10.1016/j.copbio.2016.01.008
Capolupo L, Faraco V (2016) Green methods of lignocellulose pretreatment for biorefinery development. Appl Microbiol Biotechnol 100(22):9451–9467. https://doi.org/10.1007/s00253-016-7884-y
Companhia Nacional de Abastecimento - (CONAB) (2021) Acompanhamento da Safra Brasileira. Observatório Agrícola 2(4):1–60
Terán Hilares R, de Almeida GF, Ahmed MA, Antunes FAF, da Silva SS, Han JI, dos Santos JC (2017) Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass. a parametric study. Bioresour Technol 235:301–308. https://doi.org/10.1016/j.biortech.2017.03.125
Workman JJ, Weyer L (2007) Practical guide to interpretive near-infrared spectroscopy. CRC Press
De Bhowmick G, Sarmah AK, Sen R (2018) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour Technol 247:1144–1154. https://doi.org/10.1016/j.biortech.2017.09.163
Prado CA, Antunes FAF, Rocha TM, Sánchez-Muñoz S, Barbosa FG, Terán-Hilares R, Santos JC (2021) A review on recent developments in hydrodynamic cavitation and advanced oxidative processes for pretreatment of lignocellulosic materials. Bioresour Technol 345:126458
Sałek K, Euston SR (2019) Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem 85(July):143–155. https://doi.org/10.1016/j.procbio.2019.06.027
Al-Sakkaf MK, Onaizi SA (2022) Rheology, characteristics, stability, and pH-responsiveness of biosurfactant-stabilized crude oil/water nanoemulsions. Fuel 307:121845. https://doi.org/10.1016/j.fuel.2021.121845
Song B, Zhu W, Song R, Yan F, Wang Y (2019) Exopolysaccharide from Bacillus vallismortis WF4 as an emulsifier for antifungal and antipruritic peppermint oil emulsion. Int J Biol Macromole 125:436–444. https://doi.org/10.1016/j.ijbiomac.2018.12.080
Emulsifiers Market Future on Recent Innovation 2026 Key Players (2020) Corbion, BASF SE, Lonza., Stepan Company, Akzo Nobel N.V., Estelle Chemicals Pvt. Ltd. https://www.openpr.com/news/2063526/emulsifiers-market-future-on-recent-innovation-2026-key-players. Acessed 23 January 2023.
Dhagat S, Jujjavarapu SE (2021) Simulated annealing and artificial neural network as optimization tools to enhance yields of bioemulsifier and exopolysaccharides by thermophilic Brevibacillus borstelensis. J Environ Chem Engineer 9(4):105499. https://doi.org/10.1016/j.jece.2021.105499
Bourdichon F, Casaregola S, Farrokh C, Frisvad JC, Gerds ML, Hammes WP, Harnett J, Huys G, Laulund S, Ouwehand A, Powell IB, Prajapati JB, Seto Y, Ter Schure E, Van Boven A, Vankerckhoven V, Zgoda A, Tuijtelaars S, Hansen EB (2012) Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154(3):87–97. https://doi.org/10.1016/j.ijfoodmicro.2011.12.030
Brumano LP, Antunes FAF, Souto SG, Silva G, dos Santos JC, da Silva SS (2017) Biosurfactant production by sugarcane bagasse as a renewable alternative for bioremediation process. Explor Microorg Recent Adv Appl Microbiol 15:50
Kim I, Lee I, Jeon SH, Hwang T, Han JI (2015) Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol 192:335–339. https://doi.org/10.1016/j.biortech.2015.05.038
Kim JS, Lee IK, Kim DW, Yun BS (2016) Aureosurfactin and 3-deoxyaureosurfactin, novel biosurfactants produced by Aureobasidium pullulans L3-GPY. J Antibio 69(10):759–761. https://doi.org/10.1038/ja.2015.141
Brumano LP, Antunes FAF, Souto SG, dos Santos JC, Venus J, Schneider R, da Silva SS (2017) Biosurfactant production by Aureobasidium pullulans in stirred tank bioreactor: new approach to understand the influence of important variables in the process. Bioresour Technol 243:264–272. https://doi.org/10.1016/j.biortech.2017.06.088
Velioglu Z, Ozturk UR (2014) Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solid-state fermentation. Appl Biochem Biotechnol 174(4):1354–1364. https://doi.org/10.1007/s12010-014-1136-3
Rodríguez A, Gea T, Sánchez A, Font X (2021) Agro-wastes and inert materials as supports for the production of biosurfactants by solid-state fermentation. Waste and Biomass Valoriz 12(4):1963–1976. https://doi.org/10.1007/s12649-020-01148-5
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. Biomass Anal Technol Team Lab Anal Proced 2008:1–14
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Manitchotpisit P, Price NPJ, Leathers TD, Punnapayak H (2011) Heavy oils produced by Aureobasidium pullulans. Biotechnol| Lett 33(6):1151–1157. https://doi.org/10.1007/s10529-011-0548-1
Camilios-Neto D, Bugay C, de Santana-Filho AP, Joslin T, de Souza LM, Sassaki GL, Mitchell DA, Krieger N (2011) Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl Microbiol Biotechnol 89(5):1395–1403. https://doi.org/10.1007/s00253-010-2987-3
Saur KM, Brumhard O, Scholz K, Hayen H, Tiso T (2019) A pH shift induces high-titer liamocin production in Aureobasidium pullulans. Biotechnol Prod Pro Engineering 103:4741–4752. https://doi.org/10.1007/s00253-019-09677-3
Barros FFC, Quadros CP de, Pastore GM (2008) Propriedades emulsificantes e estabilidade do biossurfactante produzido por Bacillus subtilis em manipueira. Food Sci Technol, 28(4), 979–985. https://doi.org/10.1590/s0101-20612008000400034
Tanaka M, Taniguchi M, Matsuno R, Kamikubo T (1981) Purification and properties of cellulases from Eupenicillium javanicum. J Ferment Technol 59:177–183
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods in Enzymol 160(C):87–112. https://doi.org/10.1016/0076-6879(88)60109-1
Wang C, Qi W, Liang C, Wang Q, Wang W, Wang Z, Yuan Z (2021) Impact of alkaline pretreatment condition on enzymatic hydrolysis of sugarcane bagasse and pretreatment cost. Appl Biochem Biotechnol 193(7):2087–2097. https://doi.org/10.1007/s12010-021-03530-y
Marakana PG, Dey A, Saini B (2021) Isolation of nanocellulose from lignocellulosic biomass: synthesis, characterization, modification, and potential applications. J Environ Chem Engineering 9(6):106606. https://doi.org/10.1016/j.jece.2021.106606
Vanit**da G, Nimchua T, Sukyai P (2019) Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int J Biol Macromole 122:503–516. https://doi.org/10.1016/j.ijbiomac.2018.10.191
Poletto M, Ornaghi Júnior HL, Zattera AJ (2014) Native cellulose: structure, characterization and thermal properties. Materials 7(9):6105–6119. https://doi.org/10.3390/ma7096105
Jiménez IM, Chandel AK, Marcelino PRF, Anjos V, Batesttin Costa C, Jose V, Bell M, Pereira B, da Silva SS (2020) Comparative data on effects of alkaline pretreatments and enzymatic hydrolysis on bioemulsifier production from sugarcane straw by Cutaneotrichosporon mucoides. Bioresour Technol 301:122706. https://doi.org/10.1016/j.biortech.2019.122706
Chandel AK, Antunes FAF, Anjos V, Bell MJV, Rodrigues LN, Polikarpov I, De Azevedo ER, Bernardinelli OD, Rosa CA, Pagnocca FC, da Silva SS (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7(1):1–17. https://doi.org/10.1186/1754-6834-7-63
Smith-Moritz AM, Chern M, Lao J, Sze-To WH, Heazlewood JL, Ronald PC, Vega-Sánchez ME (2011) Combining multivariate analysis and monosaccharide composition modeling to identify plant cell wall variations by Fourier transform near infrared spectroscopy. Plant Methods 7(1):10–16. https://doi.org/10.1186/1746-4811-7-26
Antunes FAF, Thomé LC, Santos JC, Ingle AP, Costa CB, Anjos VD, Bell MJV, Rosa CA, Silva SS (2021) Multi-scale study of the integrated use of the carbohydrate fractions of sugarcane bagasse for ethanol and xylitol production. Renew Energy 163:1343–1355. https://doi.org/10.1016/j.renene.2020.08.020
Hamill PG, Stevenson A, McMullan PE, Williams JP, Lewis ADR, Sudharsan S, Stevenson KE, Farnsworth KD, Khroustalyova G, Takemoto JY, Quinn JP, Rapoport A, Hallsworth JE (2020) Microbial lag phase can be indicative of, or independent from, cellular stress. Sci Rep 10(1):1–20. https://doi.org/10.1038/s41598-020-62552-4
Bera S, Banerjee T, Samanta A (2021) Evaluation of enzymatic delignification of rice straw residues for bioethanol production. Int J Renew Energy Technol 12(2):99. https://doi.org/10.1504/ijret.2021.115276
Karp SG, Faraco V, Amore A, Letti LAJ, Thomaz Soccol V, Soccol CR (2015) Statistical optimization of laccase production and delignification of sugarcane bagasse by Pleurotus ostreatus in solid-state fermentation. BioMed Res Int 2015. https://doi.org/10.1155/2015/181204
Said SD, Zaki M, Asnawi TM, Novita E (2019) Single cell protein production by a local Aspergillus niger in solid state fermentation using rice straw pulp as carbon source: effects of fermentation variables. IOP Conf Series: Mat Sci Engineer 543(1). https://doi.org/10.1088/1757-899X/543/1/012002
Pele MA, Ribeaux DR, Vieira ER, Souza AF, Luna MAC, Rodríguez DM, Andrade RFS, Alviano DS, Alviano CS, Barreto-Bergter E, Santiago ALCMA, Campos-Takaki GM (2019) Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Elec J Biotechnol 38:40–48. https://doi.org/10.1016/j.ejbt.2018.12.003
Zhu Z, Zhang F, Wei Z, Ran W, Shen Q (2013) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manage 127:96–102. https://doi.org/10.1016/j.jenvman.2013.04.017
Marques NP, de Cassia PJ, Gomes E, da Silva R, Araújo AR, Ferreira H, Rodrigues A, Dussán KJ, Bocchini DA (2018) Cellulases and xylanases production by endophytic fungi by solid-state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Industrial Crops and Products 122:66–75. https://doi.org/10.1016/j.indcrop.2018.05.022
da Silva Nunes N, Carneiro LL, de Menezes LH, de Carvalho MS, Pimentel AB, Silva TP, Pacheco CS, de Carvalho Tavares IM, Santos PH, das Chagas TP, da Silva EG (2020) Simplex-centroid design and artificial neural network-genetic algorithm for the optimization of exoglucanase production by Penicillium Roqueforti ATCC 10110 through solid-state fermentation using a blend of agroindustrial wastes. Bioenergy Res 13(4):1130–1143. https://doi.org/10.1007/s12155-020-10157-0
Zia N, Mahmood RT, Asad MJ, Hadri SH, Javaid B, Nasreen S, Safder A (2021) Fermentation of sugarcane bagasse for the production and characterization of exoglucanase by Phaeolus spadiceus. Pak J Biochem Biotechnol 2:38–48. https://doi.org/10.52700/pjbb.v2i1.33
Price NPJ, Manitchotpisit P, Vermillion KE, Bowman MJ, Leathers TD (2013) Structural characterization of novel extracellular liamocins (mannitol oils) produced by Aureobasidium pullulans strain NRRL 50380. Carbohy Res 370:24–32. https://doi.org/10.1016/j.carres.2013.01.014
Bischoff KM, Leathers TD, Price NP, Manitchotpisit P (2015) Liamocin oil from Aureobasidium pullulans has antibacterial activity with specificity for species of Streptococcus. J Antibio 68(10):642–645. https://doi.org/10.1038/ja.2015.39
Acknowledgements
The authors gratefully acknowledge the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo – Process number 2020/06323-0 and 2016/10636-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) (DS 88882.379239/2019-01) for financial support.
Data availability
Not applicable
Funding
This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo – Process number 2020/06323-0 and 2016/10636-8) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) (DS 88882.379239/2019-01) for financial support.
Author information
Authors and Affiliations
Contributions
Rogger Alessandro Mata da Costa: conceptualization, software, formal analysis, investigation, data curation, and writing—original draft preparation. Bruna Carneiro: methodology and validation. Daylin Rubio-Ribeaux: conceptualization and writing—original draft preparation. Paulo Marcelino Franco: conceptualization. Geissy de Azevedo Mendes: formal analysis. Isis Lee da Silva: formal analysis. Virgílio de Carvalho dos Anjos: software and formal analysis. Júlio César dos Santos: supervision and data curation. Till Tiso: supervision. Silvio Silvério da Silva: supervision, resource and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Alkaline pretreatment was effective for lignin removal
- Biomass pretreated was used as carbon source for the bioemulsifier production
- Aureobasidium pullulans produced bioemulsifier through solid-state fermentation
- Detection of cellulolytic enzymes confirms the use of sugarcane bagasse as carbon source
- Bioemulsifiers were identified as mannitol and arabitol-type liamocin
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Costa, R.A.M., Rubio-Ribeaux, D., Carneiro, B.C. et al. Sugarcane bagasse pretreated by different technologies used as support and carbon source in solid-state fermentation by Aureobasidium pullulans LB83 to produce bioemulsifier. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03896-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03896-5