Abstract
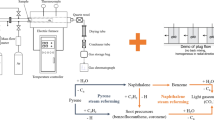
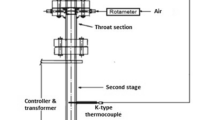
The removal of tar is conducive for improving the energy efficiency of equipment and reducing the damage caused to it. A two-stage continuous feeding apparatus was applied to examine the homogeneous conversion characteristic of biomass pyrolysis tar under the effect of steam. The tar yield could be effectively reduced from 6.68 to 2.30% by increasing steam and feedstock mass ratio (S/F) from 0 to 1.6. Furthermore, given that steam significantly affects phenyl formate (PF) cracking, Gaussian was employed to analyze its reaction mechanism. Rising the temperature from 600 to 1000 °C can improve the pyrolysis rate constant of PF by five to six orders of magnitude, and its primary products are phenol and CO. When PF reacts with H or OH radicals, the most crucial route is these radicals abstract H atom, following by releasing CO from the intermediate products. H radicals can also promote PF craking by combining with C\O atom in oxygen-containing group; among them, H radicals easily bond with the C atom.






Similar content being viewed by others
References
Wang S, Dai G, Ru B et al (2017) Influence of torrefaction on the characteristics and pyrolysis behavior of cellulose. Energy 120:864–871. https://doi.org/10.1016/j.energy.2016.11.135
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86. https://doi.org/10.1016/j.pecs.2017.05.004
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. https://doi.org/10.1021/cr900354u
Li C, Zhao X, Wang A et al (2015) Catalytic Transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624. https://doi.org/10.1021/acs.chemrev.5b00155
Sheldon RA (2014) Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem 16:950–963. https://doi.org/10.1039/C3GC41935E
Dai G, Wang K, Wang G, Wang S (2019) Initial pyrolysis mechanism of cellulose revealed by in-situ DRIFT analysis and theoretical calculation. Combust Flame 208:273–280. https://doi.org/10.1016/j.combustflame.2019.07.009
Patwardhan PR, Satrio JA, Brown RC, Shanks BH (2010) Influence of inorganic salts on the primary pyrolysis products of cellulose. Biores Technol 101:4646–4655. https://doi.org/10.1016/j.biortech.2010.01.112
Hwang H, Oh S, Cho T-S et al (2013) Fast pyrolysis of potassium impregnated poplar wood and characterization of its influence on the formation as well as properties of pyrolytic products. Biores Technol 150:359–366. https://doi.org/10.1016/j.biortech.2013.09.132
Carvalho WS, Cunha IF, Pereira MS, Ataíde CH (2015) Thermal decomposition profile and product selectivity of analytical pyrolysis of sweet sorghum bagasse: effect of addition of inorganic salts. Ind Crops Prod 74:372–380. https://doi.org/10.1016/j.indcrop.2015.05.020
Banks SW, Nowakowski DJ, Bridgwater AV (2016) Impact of potassium and phosphorus in biomass on the properties of fast pyrolysis bio-oil. Energy Fuels 30:8009–8018. https://doi.org/10.1021/acs.energyfuels.6b01044
Min Z, Asadullah M, Yimsiri P et al (2011) Catalytic reforming of tar during gasification. Part I. Steam reforming of biomass tar using ilmenite as a catalyst. Fuel 90:1847–1854. https://doi.org/10.1016/j.fuel.2010.12.039
Sueyasu T, Oike T, Mori A et al (2012) Simultaneous steam reforming of tar and steam gasification of char from the pyrolysis of potassium-loaded woody biomass. Energy Fuels 26:199–208. https://doi.org/10.1021/ef201166a
Feng D, Zhao Y, Zhang Y, Sun S (2017) Effects of H2O and CO2 on the homogeneous conversion and heterogeneous reforming of biomass tar over biochar. Int J Hydrogen Energy 42:13070–13084. https://doi.org/10.1016/j.ijhydene.2017.04.018
Effendi A, Hellgardt K, Zhang Z, Yoshida T (2005) Optimising H production from model biogas via combined steam reforming and CO shift reactions. Fuel 84:869–874. https://doi.org/10.1016/j.fuel.2004.12.011
Ren J, Cao J-P, Zhao X-Y et al (2017) Extension of catalyst lifetime by do** of Ce in Ni-loaded acid-washed Shengli lignite char for biomass catalytic gasification. Catal Sci Technol 7:5741–5749. https://doi.org/10.1039/C7CY01670K
Lu Q, Hu B, Zhang Z et al (2018) Mechanism of cellulose fast pyrolysis: the role of characteristic chain ends and dehydrated units. Combust Flame 198:267–277. https://doi.org/10.1016/j.combustflame.2018.09.025
Huang J, Liu C, Wei S et al (2010) Density functional theory studies on pyrolysis mechanism of β-d-glucopyranose. J Mol Struct (Thoechem) 958:64–70. https://doi.org/10.1016/j.theochem.2010.07.030
Zhang X, Yang W, Dong C (2013) Levoglucosan formation mechanisms during cellulose pyrolysis. J Anal Appl Pyrol 104:19–27. https://doi.org/10.1016/j.jaap.2013.09.015
Wang S, Guo X, Liang T et al (2012) Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Biores Technol 104:722–728. https://doi.org/10.1016/j.biortech.2011.10.078
Mayes HB, Broadbelt LJ (2012) Unraveling the reactions that unravel cellulose. J Phys Chem A 116:7098–7106. https://doi.org/10.1021/jp300405x
Ning H, Wu J, Ma L, Ren W (2020) Exploring the pyrolysis chemistry of prototype aromatic ester phenyl formate: reaction pathways, thermodynamics and kinetics. Combust Flame 211:337–346. https://doi.org/10.1016/j.combustflame.2019.10.002
Ning H, Liu D, Wu J et al (2018) A theoretical and shock tube kinetic study on hydrogen abstraction from phenyl formate. Phys Chem Chem Phys 20:21280–21285. https://doi.org/10.1039/C8CP02075B
Fagbemi L (2002) Pyrolysis products from different biomasses: application to the thermal cracking of tar. Fuel Energy Abstr 43:279. https://doi.org/10.1016/S0140-6701(02)86434-7
Li XT, Grace JR, Lim CJ et al (2004) Biomass gasification in a circulating fluidized bed. Biomass Bioenerg 26:171–193. https://doi.org/10.1016/S0961-9534(03)00084-9
Hu B, Huang Q, Buekens A et al (2017) Co-gasification of municipal solid waste with high alkali coal char in a three-stage gasifier. Energy Convers Manage 153:473–481. https://doi.org/10.1016/j.enconman.2017.10.026
Lu P, Huang Q, (Thanos) Bourtsalas AC et al (2018) Synergistic effects on char and oil produced by the co-pyrolysis of pine wood, polyethylene and polyvinyl chloride. Fuel 230:359–367. https://doi.org/10.1016/j.fuel.2018.05.072
Lu P, Huang Q, Chi Y et al (2019) Catalytic cracking of tar derived from the pyrolysis of municipal solid waste fractions over biochar. Proc Combust Inst 37:2673–2680. https://doi.org/10.1016/j.proci.2018.06.051
Ogliaro F, Bearpark M, Heyd J et al (2016) Gaussian 16, Revision C. 01. Gaussian. Inc, Wallingford
Lu T, Chen Q (2021) Shermo: a general code for calculating molecular thermochemistry properties. Comput Theor Chem 1200:113249. https://doi.org/10.1016/j.comptc.2021.113249
Merrick JP, Moran D, Radom L (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A 111:11683–11700. https://doi.org/10.1021/jp073974n
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Skodje R, Truhlar D, Garrett B (1982) Vibrationally adiabatic models for reactive tunneling. J Chem Phys 77:5955–5976. https://doi.org/10.1063/1.443866
Tang F, ** Y, Chi Y et al (2021) Effect of steam on the homogeneous conversion of tar contained from the co-pyrolysis of biomass and plastics. Environ Sci Pollut Res 28:68909–68919. https://doi.org/10.1007/s11356-021-15313-3
Baldwin RM, Magrini-Bair KA, Nimlos MR et al (2012) Current research on thermochemical conversion of biomass at the National Renewable Energy Laboratory. Appl Catal B 115–116:320–329. https://doi.org/10.1016/j.apcatb.2011.10.033
Williams CL, Chang C-C, Do P et al (2012) Cycloaddition of biomass-derived furans for catalytic production of renewable p-xylene. ACS Catal 2:935–939. https://doi.org/10.1021/cs300011a
Li X, Li J, Zhou G et al (2014) Enhancing the production of renewable petrochemicals by co-feeding of biomass with plastics in catalytic fast pyrolysis with ZSM-5 zeolites. Appl Catal A 481:173–182. https://doi.org/10.1016/j.apcata.2014.05.015
Sharma RK, Hajaligol MR (2003) Effect of pyrolysis conditions on the formation of polycyclic aromatic hydrocarbons (PAHs) from polyphenolic compounds. J Anal Appl Pyrol 66:123–144. https://doi.org/10.1016/S0165-2370(02)00109-2
Font Palma C (2013) Modelling of tar formation and evolution for biomass gasification: a review. Appl Energy 111:129–141. https://doi.org/10.1016/j.apenergy.2013.04.082
Fuentes-Cano D, Gómez-Barea A, Nilsson S, Ollero P (2013) Decomposition kinetics of model tar compounds over chars with different internal structure to model hot tar removal in biomass gasification. Chem Eng J 228:1223–1233. https://doi.org/10.1016/j.cej.2013.03.130
Funding
This work was supported by the National Key R&D Program of China [Grant NO. 2018YFD1100602].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, F., **, Y., Chi, Y. et al. Experimental and theoretical studies on the conversion of biomass pyrolysis tar under the effect of steam. Biomass Conv. Bioref. 14, 3917–3925 (2024). https://doi.org/10.1007/s13399-022-02638-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02638-3