Abstract
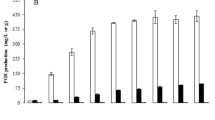
In this study, the effect of pH control and aeration on the production of Aspergillus niger inulinase from sugarbeet molasses in the bioreactor was investigated. The inulinase fermentations were performed at 30 °C temperature in 4°Bx sugarbeet molasses with a 2% (v/v) inoculation rate. The fermentation medium was only supplemented with 4.2% (w/v) yeast extract. In fermentations with aeration (1 vvm), the agitation speed was gradually increased from 200 to 600 rpm. Based on the results, it was determined that the pH-(un)controlled and non-aeration inulinase fermentations caused inadequate fungal growth and thus low inulinase and invertase-type activities, specific inulinase and invertase-type activities, and low maximum production and consumption rates. On the other hand, when the fermentation broth was aerated with 1 vvm, the inulinase and invertase-type activities, specific inulinase and invertase-type activities, and maximum production and consumption rates increased significantly under pH-controlled (5.0) and pH-uncontrolled conditions. In the fermentation with aeration, the highest inulinase and invertase-type activities were yielded as 1825.38 and 1537.88 U/mL under pH-controlled and pH-uncontrolled conditions, respectively. Also, maximum specific inulinase and invertase-type activities and maximum specific inulinase production rate were found as 5361.45 and 4371.58 U/mg and 316.71 U/mL/day with pH-uncontrolled and aerated fermentation. It was found that in both non-aeration and aeration fermentation broths, the pH control had no significant effect on enzyme production (p > 0.05). The inulinase activity/invertase-type activity ratios also varied between 0.95 and 1.56, showing that the enzyme produced is inulinase since the ratios > 10−2. Besides, with aerated fermentations, excessive and irregular fungal growth was also observed. In conclusion, aeration played a crucial role in increasing the production of inulinase and fungal growth (p < 0.05). Therefore, the best fermentation was performed under pH-uncontrolled and aeration (1 vvm) conditions (200–600 rpm agitation speed, 10 days fermentation duration, 2% (v/v) inoculum level, 30 °C temperature, 4°Bx sugarbeet molasses, and 4.2% (w/v) yeast extract). At these conditions, inulinase and invertase-type activities were 1810.76 and 1537.88 U/mL, respectively.






Similar content being viewed by others

References
Germec M, Turhan I (2020) Partial purification and characterization of Aspergillus niger inulinase produced from sugar-beet molasses in the shaking incubator and stirred-tank bioreactors. Int J Biol Macromol 164:3789–3799
Kushi R, Monti R, Contiero J (2000) Production, purification and characterization of an extracellular inulinase from Kluyveromyces marxianus var. bulgaricus. J Ind Microbiol Biotechnol 25:63–69
Pandey A, Soccol CR, Selvakumar P, Soccol VT, Krieger N, Fontana JD (1999) Recent developments in microbial inulinases. Appl Biochem Biotechnol 81(1):35–52
Singh RS, Chauhan K, Kennedy JF (2017) A panorama of bacterial inulinases: production, purification, characterization and industrial applications. Int J Biol Macromol 96:312–322
Volkov P, Sinitsyna O, Fedorova E, Rojkova A, Satrutdinov A, Zorov I, Okunev O, Gusakov A, Sinitsyn A (2012) Isolation and properties of recombinant inulinases from Aspergillus sp. Biochem Mosc 77(5):492–501
Fleming SE, GrootWassink JW, Murray ED (1979) Preparation of high-fructose syrup from the tubers of the Jerusalem artichoke (Helianthus tuberosus L.). Crit Rev Food Sci Nutr 12(1):1–28
Ettalibi M, Baratti JC (1987) Purification, properties and comparison of invertase, exoinulinases and endoinulinases of Aspergillus ficuum. Appl Microbiol Biotechnol 26(1):13–20
Germec M, Turhan I (2019) Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioprocess Biosyst Eng 42:1993–2005
Singh RS, Chauhan K, Pandey A, Larroche C, Kennedy JF (2018) Purification and characterization of two isoforms of exoinulinase from Penicillium oxalicum BGPUP-4 for the preparation of high fructose syrup from inulin. Int J Biol Macromol 118:1974–1983
Neagu C, Bahrim G (2011) Inulinases-a versatile tool for biotechnology. Innov Roman Food Biotechnol 9:1
Kango N, Jain SC (2011) Production and properties of microbial inulinases: recent advances. Food Biotechnol 25(3):165–212
Vandamme EJ, Derycke DG (1983) Microbial inulinases: fermentation process, properties, and applications. Adv Appl Microbiol 29:139–176
Pitt JI, Hocking AD (2009) Aspergillus and related teleomorphs. In: Fungi and food spoilage. Springer, pp 275–337
Schuster E, Dunn-Coleman N, Frisvad J, van Dijck P (2002) On the safety of Aspergillus niger -a review. Appl Microbiol Biotechnol 59(4):426–435. https://doi.org/10.1007/s00253-002-1032-6
Germec M, Gürler HN, Ozcan A, Erkan SB, Karahalil E, Turhan I (2020) Medium optimization and kinetic modeling for the production of Aspergillus niger inulinase. Bioprocess Biosyst Eng 43(2):217–232
Germec M, Ozcan A, Turhan I (2019) Bioconversion of wheat bran into high value-added products and modelling of fermentations. Ind Crop Prod 139:111565
Germec M, Turhan I (2020) Enhanced production of Aspergillus niger inulinase from sugar beet molasses and its kinetic modeling. Biotechnol Lett 42(10):1939–1955
Germec M, Turhan I (2020) Thermostability of Aspergillus niger inulinase from sugar beet molasses in the submerged fermentation and determination of its kinetic and thermodynamic parameters. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00809-8
Gürler HN, Germec M, Turhan I (2020) The inhibition effect of phenol on the production of Aspergillus niger inulinase and its modeling. J Food Process Preserv:e14522. https://doi.org/10.1111/jfpp.14522
Ilgın M, Germec M, Turhan I (2020) Inulinase production and mathematical modeling from carob extract by using Aspergillus niger. Biotechnol Prog 36. https://doi.org/10.1002/btpr.2919
Ilgın M, Germec M, Turhan I (2020) Statistical and kinetic modeling of Aspergillus niger inulinase fermentation from carob extract and its partial concentration. Ind Crop Prod 156:112866
Singh R, Singh R (2017) Inulinases. In: Pandey A, Negi S, Soccol CR (eds) Current developments in biotechnology and bioengineering: production, isolation and purification of industrial products. Elsevier, Amsterdam, pp 423–446
Wang L, Huang Y, Long X, Meng X, Liu Z (2011) Cloning of exoinulinase gene from Penicillium janthinellum strain B01 and its high-level expression in Pichia pastoris. J Appl Microbiol 111(6):1371–1380
Zhang S, Yang F, Wang Q, Hua Y, Zhao ZK (2012) High-level secretory expression and characterization of the recombinant Kluyveromyces marxianus inulinase. Process Biochem 47(1):151–155
Singh RS, Chauhan K (2018) Production, purification, characterization and applications of fungal inulinases. Curr Biotechnol 7(3):242–260
Kowalska A, Antecka A, Owczarz P, Bizukojć M (2017) Inulinolytic activity of broths of Aspergillus niger ATCC 204447 cultivated in shake flasks and stirred tank bioreactor. Eng Life Sci 17(9):1006–1020
Ongen-Baysal G, Sukan SS (1996) Production of inulinase by mixed culture of Aspergillus niger and Kluyveromyces marxianus. Biotechnol Lett 18(12):1431–1434
Ongen-Baysal G, Sukan SS, Vassilev N (1994) Production and properties of inulinase from Aspergillus niger. Biotechnol Lett 16(3):275–280. https://doi.org/10.1007/bf00134625
Singh R, Singh R (2014) Response surface optimization of endoinulinase production from a cost effective substrate by Bacillus safensis AS-08 for hydrolysis of inulin. Biocatal Agricult Biotechnol 3(4):365–372
Tang Y-J, Zhong J-J (2003) Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enzym Microb Technol 32(3-4):478–484
de Oliveira Lino FS, Basso TO, Sommer MOA (2018) A synthetic medium to simulate sugarcane molasses. Biotechnol Biofuels 11(1):221
Demirci A, Öziyci HR, Karhan M, Turkenburg JP (2014) Fermentasyon besiyeri. In: Turhan I (ed) Endüstriyel Mikrobiyolojiye Giriş. Palme Yayıncılık, Ankara, pp 86–93
Yuan X-L, Goosen C, Kools H, van der Maarel MJ, van den Hondel CAJ, Dijkhuizen L, Ram AF (2006) Database mining and transcriptional analysis of genes encoding inulin-modifying enzymes of Aspergillus niger. Microbiology 152(10):3061–3073
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Bender JP, Mazutti MA, de Oliveira D, Di Luccio M, Treichel H (2006) Inulinase production by Kluyveromyces marxianus NRRL Y-7571 using solid state fermentation. Appl Biochem Biotechnol 132(1-3):951–958
Kalil S, Suzan R, Mougeri F, Rodrigues M (2001) Optimization of inulinase production by Kluyveromyces marxianus using factorial design. Appl Biochem Biotechnol 94:257–264
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Germec M, Turhan I, Karhan M, Demirci A (2015) Ethanol production via repeated-batch fermentation from carob pod extract by using Saccharomyces cerevisiae in biofilm reactor. Fuel 161:304–311
Shuler M, Kargi F (2017) How cells grow. In: Shuler M, Kargi F (eds) Bioprocess engineering: basic concepts. Prentice Hall, Upper Saddle River, pp 155–206
Silva-Santisteban B, Filho F (2005) Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus. Enzym Microb Technol 36:717–724
Santharam L, Easwaran SN, Subramanian Mohanakrishnan A, Mahadevan S (2019) Effect of aeration and agitation on yeast inulinase production: a biocalorimetric investigation. Bioprocess Biosyst Eng 42:1009–1021. https://doi.org/10.1007/s00449-019-02101-0
Singh RS, Chauhan K, Pandey A (2019) Influence of aeration, agitation and process duration on fungal inulinase production from paneer whey in a stirred tank reactor. Bioresour Technol Rep 8:100343
Singh RS, Chauhan K (2018) Sequential statistical optimization of lactose-based medium and process variables for inulinase production from Penicillium oxalicum BGPUP-4. 3 Biotech 8(1):38
Bakri Y, Mekaeel A, Koreih A (2011) Influence of agitation speeds and aeration rates on the xylanase activity of Aspergillus niger SS7. Braz Arch Biol Technol 54(4):659–664
Chen G-J, Yang J-K, Peng X-B, He J-R (2016) High-level secretory expression of Aspergillus exo-inulinase and its use in the preparation of fructose syrup from inulin. J Mol Catal B Enzym 133:S543–S551
Chesini M, Neila LP, de la Parra DF, Rojas NL, Contreras Esquivel JC, Cavalitto SF, Ghiringhelli PD, Hours RA (2013) Aspergillus kawachii produces an inulinase in cultures with yacon (Smallanthus sonchifolius) as substrate. Electron J Biotechnol 16(3):8–8
Viswanathan P, Kulkarni P (1995) Saussurea lappa (kuth) as a new source of inulin for fermentative production of inulinase in a laboratory stirred fermenter. Bioresour Technol 52(2):181–184
Mazutti MA, Corazza ML, Maugeri Filho F, Rodrigues MI, Corazza FC, Treichel H (2009) Inulinase production in a batch bioreactor using agroindustrial residues as the substrate: experimental data and modeling. Bioprocess Biosyst Eng 32(1):85–95
Treichel H, Mazutti MA, Maugeri F, Rodrigues MI (2009) Use of a sequential strategy of experimental design to optimize the inulinase production in a batch bioreactor. J Ind Microbiol Biotechnol 36(7):895–900
Mansouri S, Houbraken J, Samson R, Frisvad JC, Christensen M, Tuthill D, Koutaniemi S, Hatakka A, Lankinen P (2013) Penicillium subrubescens, a new species efficiently producing inulinase. Antonie Van Leeuwenhoek 103(6):1343–1357
Dinarvand M, Rezaee M, Foroughi M (2017) Optimizing culture conditions for production of intra and extracellular inulinase and invertase from Aspergillus niger ATCC 20611 by response surface methodology (RSM). Braz J Microbiol 48(3):427–441
Trivedi S, Divecha J, Shah A (2012) Optimization of inulinase production by a newly isolated Aspergillus tubingensis CR16 using low cost substrates. Carbohydr Polym 90(1):483–490
Zhang T, Gong F, Peng Y, Chi Z (2009) Optimization for high-level expression of the Pichia guilliermondii recombinant inulinase in Pichia pastoris and characterization of the recombinant inulinase. Process Biochem 44(12):1335–1339
Makino Y, Treichel H, Mazutti MA, Maugeri F, Rodrigues MI (2009) Inulinase bio-production using agroindustrial residues: screening of microorganisms and process parameters optimization. J Chem Technol Biotechnol 84(7):1056–1062
Declarations
a
Conflict of interest
The authors declare no competing interests.
Funding
This work was supported by the Akdeniz University Research Foundation (Grant number FDK-2019-4761).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Germec, M., Turhan, I. Effect of pH control and aeration on inulinase production from sugarbeet molasses in a bench-scale bioreactor. Biomass Conv. Bioref. 13, 4727–4739 (2023). https://doi.org/10.1007/s13399-021-01436-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01436-7