Abstract
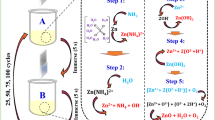
Atomic layer deposition (ALD) is a promising technique for fabricating high-quality thin films. For improving the process conditions and material quality of ALD, understanding the surface chemical mechanisms at the molecular level is important as the entire ALD process is based on the reactions of precursors on the substrate surfaces. Zinc oxynitride (ZnON) is gaining significant research interest as a p-type semiconductor material. Although the ALD of ZnON can be performed by dosing H2O and NH3 as oxygen and nitrogen sources, respectively, the elemental ratio of O and N in the deposited film differs considerably from that in the gaseous sources. In this study, the surface reactions of ZnON ALD are analyzed employing density functional theory calculations. All the ALD surface reactions of ZnO and ZnN are facile and expected to occur rapidly. However, the substitution of a surface *NH2 by H2O to form *OH is preferred, whereas the inverse reaction is implausible. We propose that the differences in the reactivity could originate from the higher bond energy of Zn–O than that of Zn–N.
Graphical Abstract






Similar content being viewed by others
References
Street, R.A.: Thin-film transistors. Adv. Mater. 21, 2007–2022 (2009)
Fortunato, E., Barquinha, P., Martins, R.: Oxide semiconductor thin-film transistors: a review of recent advances. Adv. Mater. 24, 2945–2986 (2012)
Park, J.W., Kang, B.H., Kim, H.J.: A review of low-temperature solution-processed metal oxide thin-film transistors for flexible electronics. Adv. Funct. Mater. 30, 1904632 (2020)
Baek, I.-H., et al.: High-performance thin-film transistors of quaternary indium–zinc–tin oxide films grown by atomic layer deposition. ACS Appl. Mater. Interfaces 11, 14892–14901 (2019)
Hur, J.S., et al.: High-performance thin-film transistor with atomic layer deposition (ALD)-derived indium-gallium oxide channel for back-end-of-line compatible transistor applications: cation combinatorial approach. ACS Appl. Mater. Interfaces 14, 48857–48867 (2022)
Hong, T., Kim, Y.-S., Choi, S.-H., Lim, J.H., Park, J.-S.: Exploration of chemical composition of In–Ga–Zn–O system via PEALD technique for optimal physical and electrical properties. Adv. Electron. Mater. 9, 2201208 (2023)
Burgess, C.H.: Review of tailoring ZnO for optoelectronics through atomic layer deposition experimental variables. Mater. Sci. Technol. 33, 809–821 (2017)
Wang, M., et al.: High-performance flexible ZnO thin-film transistors by atomic layer deposition. IEEE Electron Device Lett. 40, 419–422 (2019)
Saha, J.K., Billah, M.M., Jang, J.: Triple-stack ZnO/AlZnO/YZnO heterojunction oxide thin-film transistors by spray pyrolysis for high mobility and excellent stability. ACS Appl. Mater. Interfaces 13, 37350–37362 (2021)
Kim, H.-D., Kim, J.H., Jang, S.C., Nahm, H.-H., Kim, H.-S.: Deterministic role of fluorine incorporation in the amorphous Zn–O–N semiconductors: first-principles and experimental studies. AIP Adv. 11, 105102 (2021)
Kim, H.-D., et al.: Nonvolatile high-speed switching Zn-O-N thin-film transistors with a bilayer structure. ACS Appl. Mater. Interfaces 14, 13490–13498 (2022)
Park, J., et al.: The effects of active layer thickness and annealing conditions on the electrical performance of ZnON thin-film transistors. J. Alloys Compd. 688, 666–671 (2016)
Cho, M.H., Choi, C.H., Jeong, J.K.: Recent progress and perspectives on atomic-layer-deposited semiconducting oxides for transistor applications. J. Soc. Inf. Disp. 30, 175–197 (2022)
Macco, B., Kessels, W.M.M.: Atomic layer deposition of conductive and semiconductive oxides. Appl. Phys. Rev. 9, 041313 (2022)
Kim, H.-M., Kim, D.-G., Kim, Y.-S., Kim, M., Park, J.-S.: Atomic layer deposition for nanoscale oxide semiconductor thin film transistors: review and outlook. Int. J. Extreme Manuf. 5, 012006 (2023)
Mackus, A.J.M., Schneider, J.R., MacIsaac, C., Baker, J.G., Bent, S.F.: Synthesis of doped, ternary, and quaternary materials by atomic layer deposition: a review. Chem. Mater. 31, 1142–1183 (2019)
Coll, M., Napari, M.: Atomic layer deposition of functional multicomponent oxides. APL Mater. 7, 110901 (2019)
Tripathi, T.S., Karppinen, M.: Mixed-anion compounds: an unexplored playground for ALD fabrication. Adv. Mater. Interfaces 8, 2100146 (2021)
Lujala, V., Skarp, J., Tammenmaa, M., Suntola, T.: Atomic layer epitaxy growth of doped zinc oxide thin films from organometals. Appl. Surf. Sci. 82–83, 34–40 (1994)
Sinha, S., Sarkar, S.K.: Atomic layer deposition of textured zinc nitride thin films. RSC Adv. 4, 47177–47183 (2014)
Hsu, C.T.: Growth of ZnSxSe1−x layers on Si substrates by atomic layer epitaxy. Mater. Chem. Phys. 58, 6–11 (1999)
Mullings, M.N., et al.: Thin film characterization of zinc tin oxide deposited by thermal atomic layer deposition. Thin Solid Films 556, 186–194 (2014)
Lancaster, D.K., Sun, H., George, S.M.: Atomic layer deposition of Zn(O, S) Alloys Using diethylzinc with H2O and H2S: effect of exchange reactions. J. Phys. Chem. C 121, 18643–18652 (2017)
Choi, J.-W., et al.: Tin oxysulfide composite thin films based on atomic layer deposition of tin sulfide and tin oxide using Sn(dmamp)2 as Sn precursor. Ceram. Int. 46, 5109–5118 (2020)
Choi, S., et al.: Growth of Al-rich AlGaN thin films by purely thermal atomic layer deposition. J. Alloys Compd. 854, 157186 (2021)
Lee, C., Lim, J.: Dependence of the electrical properties of the ZnO thin films grown by atomic layer epitaxy on the reactant feed sequence. J. Vac. Sci. Technol. A 24, 1031–1035 (2006)
Levy, D.H., Freeman, D., Nelson, S.F., Cowdery-Corvan, P.J., Irving, L.M.: Stable ZnO thin film transistors by fast open air atomic layer deposition. Appl. Phys. Lett. 92, 192101 (2008)
Lim, S.J., et al.: Atomic layer deposition ZnO: N Thin film transistor: the effects of N concentration on the device properties. J. Electrochem. Soc. 157, H214 (2009)
Chien, J.-F., Chen, C.-H., Shyue, J.-J., Chen, M.-J.: Local electronic structures and electrical characteristics of well-controlled nitrogen-doped ZnO thin films prepared by remote plasma in situ atomic layer do**. ACS Appl. Mater. Interfaces 4, 3471–3475 (2012)
Kim, S.H., et al.: Growth enhancement and nitrogen loss in ZnOxNy low-temperature atomic layer deposition with NH3. J. Phys. Chem. C 119, 23470–23477 (2015)
Guziewicz, E., et al.: Abundant acceptor emission from nitrogen-doped ZnO films prepared by atomic layer deposition under oxygen-rich conditions. ACS Appl. Mater. Interfaces 9, 26143–26150 (2017)
Ding, X., et al.: Nitrogen-doped ZnO film fabricated via rapid low-temperature atomic layer deposition for high-performance ZnON transistors. IEEE Trans. Electron Dev 65, 3283–3290 (2018)
Pore, V., Heikkilä, M., Ritala, M., Leskelä, M., Areva, S.: Atomic layer deposition of TiO2−xNx thin films for photocatalytic applications. J. Photochem. Photobiol. Chem. 177, 68–75 (2006)
Parashar, P.K., Kinnunen, S.A., Sajavaara, T., Toppari, J.J., Komarala, V.K.: Thermal atomic layer deposition of AlOxNy thin films for surface passivation of nano-textured flexible silicon. Sol. Energy Mater. Sol. Cells 193, 231–236 (2019)
Richey, N.E., de Paula, C., Bent, S.F.: Understanding chemical and physical mechanisms in atomic layer deposition. J. Chem. Phys. 152, 040902 (2020)
Li, J., Chai, G., Wang, X.: Atomic layer deposition of thin films: from a chemistry perspective. Int. J. Extreme Manuf. 5, 032003 (2023)
Ren, J.: Initial growth mechanism of atomic layer deposition of ZnO on the hydroxylated Si(100)−2×1: a density functional theory study. Appl. Surf. Sci. 255, 5742–5745 (2009)
Dong, L., et al.: Initial reaction mechanism of nitrogen-doped zinc oxide with atomic layer deposition. Thin Solid Films 517, 4355–4359 (2009)
Tanskanen, J.T., Hägglund, C., Bent, S.F.: Correlating growth characteristics in atomic layer deposition with precursor molecular structure: the case of zinc tin oxide. Chem. Mater. 26, 2795–2802 (2014)
Weckman, T., Laasonen, K.: Atomic layer deposition of zinc oxide: diethyl zinc reactions and surface saturation from first-principles. J. Phys. Chem. C 120, 21460–21471 (2016)
Weckman, T., Laasonen, K.: Atomic layer deposition of zinc oxide: study on the water pulse reactions from first-principles. J. Phys. Chem. C 122, 7685–7694 (2018)
Van, N., Thi, T., Ansari, A.S., Shong, B.: Surface chemical reactions during atomic layer deposition of ZnO, ZnS, and Zn (O, S). J. Vac. Sci. Technol. A 37(2), 98 (2019)
Frisch, M. J. et al. (2016) Gaussian 16.
Neese, F.: Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2018)
Klaus, J.W., George, S.M.: Atomic layer deposition of SiO2 at room temperature using NH3-catalyzed sequential surface reactions. Surf. Sci. 447, 81–90 (2000)
Jung, S.-H., Kang, S.-W.: Formation of TiO2 thin films using NH3 as catalyst by metalorganic chemical vapor deposition. Jpn. J. Appl. Phys. 40, 3147 (2001)
Chen, S., Fang, G., Qian, X., Li, A., Ma, J.: Influence of Alkalinity and steric hindrance of lewis-base catalysts on atomic layer deposition of SiO2. J. Phys. Chem. C 115, 23363–23373 (2011)
Fang, G., Chen, S., Li, A., Ma, J.: Surface pseudorotation in lewis-base-catalyzed atomic layer deposition of SiO2: static transition state search and born-oppenheimer molecular dynamics simulation. J. Phys. Chem. C 116, 26436–26448 (2012)
Luo, Y.R.: Comprehensive Handbook of Chemical Bond Energies. CRC Press (2007)
Wriedt, H.A.: The N−Zn (Nitrogen-Zinc) system. Bull. Alloy Phase Diagr. 9, 247–251 (1988)
Dean, J.A.: Lange’s Handbook of Chemistry. McGraw-Hill (1998)
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT, RS-2023-00210186). This work was supported by the Technology Innovation Program (Public-private joint investment semiconductor R&D program (K-CHIPS) to foster high-quality human resources) (RS-2023-00236667, High performance Ru-TiN interconnects via high temperature atomic layer deposition (ALD) and development on new interconnect materials based on ALD) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea) (1415187401). This work was supported by the National Supercomputing Center with supercomputing resources including technical support (KSC-2022-CRE-0280).
Funding
The Funding was provided by National Research Foundation of Korea, (RS-2023-00210186), Bonggeun Shong, Technology Innovation Program, (RS-2023-00236667), Bonggeun Shong
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing Interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ngoc Van, T.T., Shong, B. Surface Chemical Reactions During Atomic Layer Deposition of Zinc Oxynitride (ZnON). Electron. Mater. Lett. 20, 500–507 (2024). https://doi.org/10.1007/s13391-023-00467-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-023-00467-8