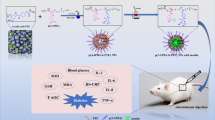
Abstract
Puerarin (Pue) is a naturally bioactive compound with many potential functions in regulating blood glucose and lipid metabolism. However, the low bioavailability and rapid elimination in vivo limit the application of Pue in diabetic treatment. Here, we developed a metal-polyphenol-functionalized microgel to effectively deliver Pue in vivo and eventually alleviate the onset of diabetes. Pue was initially encapsulated in alginate beads through electrospray technology, and further immersed in Fe3+ and tannic acid solution from tannic acid (TA)–iron (Fe) coatings (TF). These constructed Pue@SA-TF microgels exhibited uniform spheres with an average size of 367.89 ± 18.74 µm and high encapsulation efficiency of Pue with 61.16 ± 1.39%. In vivo experiments proved that compared with free Pue and microgels without TF coatings, the biological distribution of Pue@SA-TF microgels specifically accumulated in the small intestine, prolonged the retention time of Pue, and achieved a high effectiveness in vivo. Anti-diabetic experimental results showed that Pue@SA-TF microgels significantly improved the levels of blood glucose, blood lipid, and oxidative stress in diabetic mice. Meanwhile, histopathological observations indicated that Pue@SA-TF microgels could significantly alleviate the damage to the liver, kidney, and pancreas in diabetic mice. Our study provided an effective strategy for oral delivery of Pue and achieved high anti-diabetic efficacy.
Graphical Abstract







Similar content being viewed by others
Data availability
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arya A, Jamil Al-Obaidi M, Shahid N, Bin Noordin M, Looi C, Wong W, et al. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: a mechanistic study. Food Chem Toxicol. 2014;71:183–96. https://doi.org/10.1016/j.fct.2014.06.010.
Liu M, Song X, Zhang J, Zhang C, Gao Z, Li S, et al. Protective effects on liver, kidney and pancreas of enzymatic-and acidic-hydrolysis of polysaccharides by spent mushroom compost (Hypsizigus marmoreus). Sci Rep-UK. 2017;7(1):43212. https://doi.org/10.1038/srep43212.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan B, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pr. 2022;183:109119. https://doi.org/10.1016/j.diabres.2021.109119.
Fan M, Zhang X, Zhao Y, Zhi J, Xu W, Yang Y, et al. Mn (II)-mediated self-assembly of tea polysaccharide nanoparticles and their functional role in mice with type 2 diabetes. ACS Appl Mater Interfaces. 2022;14(27):30607–17. https://doi.org/10.1021/acsami.2c07488.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and co** strategies. Diabetes Obes Metab. 2007;9(6):799–812. https://doi.org/10.1111/j.1463-1326.2006.00686.x.
Madić V, Petrović A, Jušković M, Jugović D, Djordjević L, Stojanović G, et al. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J Ethnopharmacol. 2021;265:113210. https://doi.org/10.1016/j.jep.2020.113210.
He M, Yu P, Hu Y, Zhang J, He M, Nie C, et al. Erythrocyte-membrane-enveloped biomineralized metal–organic framework nanoparticles enable intravenous glucose-responsive insulin delivery. ACS Appl Mater Interfaces. 2021;13(17):19648–59. https://doi.org/10.1021/acsami.1c01943.
Marton L, Pescinini-e-Salzedas L, Camargo M, Barbalho S, Haber J, Sinatora R, et al. The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol. 2021;12:669448. https://doi.org/10.3389/fendo.2021.669448.
Öztürk E, Arslan A, Yerer M, Bishayee A. Resveratrol and diabetes: a critical review of clinical studies. Biomed Pharmacother. 2017;95:230–4. https://doi.org/10.1016/j.biopha.2017.08.070.
Wang Y, Liu H, Zheng M, Yang Y, Ren H, Kong Y, et al. Berberine slows the progression of prediabetes to diabetes in zucker diabetic fatty rats by enhancing intestinal secretion of glucagon-like peptide-2 and improving the gut microbiota. Front Endocrinol. 2021;12:609134. https://doi.org/10.3389/fendo.2021.609134.
Noh J, Yang H, Jun M, Lee B. Puerarin attenuates obesity-induced inflammation and dyslipidemia by regulating macrophages and TNF-Alpha in obese mice. biomedicine. 2022;10(1):175. https://doi.org/10.3390/biomedicines10010175.
Chen X, Yu J, Shi J. Management of diabetes mellitus with puerarin, a natural isoflavone from pueraria lobata. Am J Chinese Med. 2018;46(8):1771–89. https://doi.org/10.1142/s0192415x185.
Deng X, Zhang H, Wang G, Xu D, Zhang W, Wang Q, et al. Colon-specific microspheres loaded with puerarin reduce tumorigenesis and metastasis in colitis-associated colorectal cancer. Int J Pharmaceut. 2019;570(C):118644. https://doi.org/10.1016/j.ijpharm.2019.118644.
Bohr A, Kristensen J, Stride E, Dyas M, Edirisinghe M. Preparation of microspheres containing low solubility drug compound by electrohydrodynamic spraying. Int J Pharmaceut. 2011;412(1):59–67. https://doi.org/10.1016/j.ijpharm.2011.04.005.
Pasparakis G, Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int J Pharmaceut. 2006;323(1):34–42. https://doi.org/10.1016/j.ijpharm.2006.05.054.
Wang Q, Wang G, Zhou J, Gao L, Cui Y. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharmaceut. 2016;515(1–2):176–85. https://doi.org/10.1016/j.ijpharm.2016.10.002.
Bi Y, Lin Z, Deng S. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mat Sci Eng C. 2019;100:576–83. https://doi.org/10.1016/j.msec.2019.03.040.
Sheng Y, Gao J, Yin Z, Kang J, Kong Y. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohyd Polym. 2021;269:118325. https://doi.org/10.1016/j.carbpol.2021.118325.
Hou J, Gao L, Meng F, Cui Y. Mucoadhesive microparticles for gastroretentive delivery: preparation, biodistribution and targeting evaluation. Mar Drugs. 2014;12(12):5764–87. https://doi.org/10.3390/md12125764.
Long Y, **ao L, Cao Q, Shi X, Wang Y. Efficient incorporation of diverse components into metal organic frameworks via metal phenolic networks. Chem Commun. 2017;53(78):10831–4. https://doi.org/10.1039/C7CC05710E.
Bartzoka E, Lange H, Poce G, Crestini C. Stimuli-responsive tannin–FeIII hybrid microcapsules demonstrated by the active release of an anti-tuberculosis agent. Chemsuschem. 2018;11(22):3975–91. https://doi.org/10.1002/cssc.201801546.
Kunyanga C, Imungi J, Okoth M, Momanyi C, Biesalski H, Vadivel V. Antioxidant and antidiabetic properties of condensed tannins in acetonic extract of selected raw and processed indigenous food ingredients from kenya. J Food Sci. 2011;76(4):C560–7. https://doi.org/10.1111/j.1750-3841.2011.02116.x.
Guo J, ** Y, Ejima H, Alt K, Meissner M, Richardson J, et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew Chem Int Ed Engl. 2014;53(22):5546–51. https://doi.org/10.1002/anie.201311136.
Lee J, Cho H, Choi J, Kim D, Hong D, Park J, et al. Chemical sporulation and germination: cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid–FeIII complex. Nanoscale. 2015;7(45):18918–22. https://doi.org/10.1039/C5NR05573C.
Ejima H, Richardson J, Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–48. https://doi.org/10.1016/j.nantod.2016.12.012.
Kim B, Han S, Lee K, Choi I. Biphasic supramolecular self-assembly of ferric ions and tannic acid across interfaces for nanofilm formation. Adv Mater. 2017;29(28):1700784. https://doi.org/10.1002/adma.201700784.
Ejima H, Richardson J, Liang K, Best J, van Koeverden M, Such G, et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154–7. https://doi.org/10.1126/science.1237265.
Regmi S, Pathak S, Nepal M, Shrestha P, Park J, Kim J, et al. Inflammation-triggered local drug release ameliorates colitis by inhibiting dendritic cell migration and Th1/Th17 differentiation. J Control Release. 2019;316:138–49. https://doi.org/10.1016/j.jconrel.2019.11.001.
Wang X, Gu H, Zhang H, **an J, Li J, Fu C, et al. Oral core–shell nanoparticles embedded in hydrogel microspheres for the efficient site-specific delivery of magnolol and enhanced antiulcerative colitis therapy. ACS Appl Mater Interfaces. 2021;13(29):33948–61. https://doi.org/10.1021/acsami.1c09804.
Halder M, Bhatia Y, Singh Y. Self-assembled di- and tripeptide gels for the passive entrapment and pH-responsive, sustained release of an antidiabetic drug, glimepiride. Biomater Sci-UK. 2022;10(9):2248–62. https://doi.org/10.1039/d2bm00344a.
Rodríguez-Rodríguez R, Velasquillo-Martínez C, Knauth P, López Z, Moreno-Valtierra M, Bravo-Madrigal J, et al. Sterilized chitosan-based composite hydrogels: physicochemical characterization and in vitro cytotoxicity. J Biomed Mater Res A. 2020;108(1):81–93. https://doi.org/10.1002/jbm.a.36794.
Tekula S, Khurana A, Anchi P, Godugu C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed Pharmacother. 2018;106:1428–40. https://doi.org/10.1016/j.biopha.2018.07.090.
Fan L, Niu H, Zhao L, Yao R, He X, Lu B, et al. Purendan alleviates non-alcoholic fatty liver disease in aged type 2 diabetic rats via regulating mTOR/S6K1/SREBP-1c signaling pathway. Biomed Pharmacother. 2022;148:112697. https://doi.org/10.1016/j.biopha.2022.112697.
Lee H, Nguyen D, Kim N, Han S, Hong Y, Yun G, et al. Enzyme-mediated kinetic control of Fe3+–tannic acid complexation for interface engineering. ACS Appl Mater Interfaces. 2021;13(44):52385–94. https://doi.org/10.1021/acsami.1c15503.
Dong J, Chen W, Feng J, Liu X, Xu Y, Wang C, et al. Facile, smart, and degradable metal–organic framework nanopesticides gated with FeIII-tannic acid networks in response to seven biological and environmental stimuli. ACS Appl Mater Interfaces. 2021;13(16):19507–20. https://doi.org/10.1021/acsami.1c04118.
Kozlovskaya V, Kharlampieva E, Drachuk I, Cheng D, Tsukruk VV. Responsive microcapsule reactors based on hydrogen-bonded tannic acid layer-by-layer assemblies. Soft Matter. 2010;6(15):3596–608. https://doi.org/10.1039/B927369G.
Li Y, Wang C, Luan Y, Liu W, Chen T, Liu P, et al. Preparation of pH-responsive cellulose nanofibril/sodium alginate based hydrogels for drug release. J Appl Polym Sci. 2022;139(7):51647. https://doi.org/10.1002/app.51647.
Chen J, Li J, Zhou J, Lin Z, Cavalieri F, Czuba-Wojnilowicz E, et al. Metal–phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano. 2019;13(10):11653–64. https://doi.org/10.1021/acsnano.9b05521.
Zhang C, Peng S, Hong S, Chen Q, Zeng X, Rong L, et al. Biomimetic carbon monoxide nanogenerator ameliorates streptozotocin induced type 1 diabetes in mice. Biomaterials. 2020;245:119986. https://doi.org/10.1016/j.biomaterials.2020.119986.
Zhuang Y, Yang X, Li Y, Chen Y, Peng X, Yu L, et al. Sustained release strategy designed for lixisenatide delivery to synchronously treat diabetes and associated complications. ACS Appl Mater Interfaces. 2019;11(33):29604–18. https://doi.org/10.1021/acsami.9b10346.
**e Y, Bowe B, Li T, **an H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93(3):741–52. https://doi.org/10.1016/j.kint.2017.08.033.
Matsue Y, van der Meer P, Damman K, Metra M, Connor C, Ponikowski P, et al. Blood urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart. 2017;103(6):407–14. https://doi.org/10.1136/heartjnl-2016-310112.
Lan Q, Zheng L, Zhou X, Wu H, Buys N, Liu Z, et al. The value of blood urea nitrogen in the prediction of risks of cardiovascular disease in an older population. Front Cardiovasc Med. 2021;8:614117. https://doi.org/10.3389/fcvm.2021.614117.
Schneider C, Coll B, Jick S, Meier C. Doubling of serum creatinine and the risk of cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes mellitus: a cohort study. Clin Epidemiol. 2016;8:177–84. https://doi.org/10.2147/clep.S107060.
Liu M, Wang Z, Feng D, Shang Y, Li X, Liu J, et al. An insulin-inspired supramolecular hydrogel for prevention of type 1 diabetes. Adv Sci. 2021;8(10):2003599. https://doi.org/10.1002/advs.202003599.
Xu D, Guo X, Zeng Z, Wang Y, Qiu J. Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. 2021;12(6):2726–40. https://doi.org/10.1039/D0FO02761H.
Wu J, Williams G, Branford-White C, Li H, Li Y, Zhu L. Liraglutide-loaded poly (lactic-co-glycolic acid) microspheres: preparation and in vivo evaluation. Eur J of Pharm Sci. 2016;92:28–38. https://doi.org/10.1016/j.ejps.2016.06.018.
Zhang Y, Huang N, Yan F, ** H, Zhou S, Shi J, et al. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav Brain Res. 2018;339:57–65. https://doi.org/10.1016/j.bbr.2017.11.015.
Mandard S, Stienstra R, Escher P, Tan N, Kim I, Gonzalez F, et al. Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cell Mol Life Sci. 2007;64(9):1145–57. https://doi.org/10.1007/s00018-007-7006-1.
Yang J, Zhang T, Dong Z, Shi H, Xu J, Mao X, et al. Dietary supplementation with exogenous sea-cucumber-derived ceramides and glucosylceramides alleviates insulin resistance in high-fructose-diet-fed rats by upregulating the IRS/PI3K/Akt signaling pathway. J Agric Food Chem. 2021;69(32):9178–87. https://doi.org/10.1021/acs.jafc.0c06831.
Acknowledgements
The authors appreciate the staff from the National Engineering Research Center of Seafood of Dalian Polytechnic University for their technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (32172208) and Central Funds Guiding the Local Science and Technology Development of China (2020JH6/10500002).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and writing—original draft, S H Li; methodology and investigation, Y F Li; writing review and editing, D Wu; data curation and writing editing, Y Xu; methodology and investigation, H J Yan; funding acquisition and writing editing, J N Hu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures of this investigation were approved by the Experimental Animal Ethics Committee of National Engineering Research Center of Seafood of Dalian Polytechnic University (Dalian, China, Animal JN. No DLPU2022010).
Consent to publication
All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13346_2023_1428_MOESM1_ESM.docx
The characterization images of SA and SA-TF microgels, Fig. S1. Microscopy images of SA microgels in simulated digestive solutions at various stages, Fig. S2A. Swelling indexes of SA microgels and SA-TF microgels, Fig. S2B and C. The synthesis process and biocompatibility of Pue@SA-TF microgels, results of protein expression by Western blotting, Fig S3. (DOCX 1379 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Sh., Li, Yf., Wu, D. et al. Metal-polyphenol microgels for oral delivery of puerarin to alleviate the onset of diabetes. Drug Deliv. and Transl. Res. 14, 757–772 (2024). https://doi.org/10.1007/s13346-023-01428-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01428-2