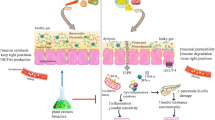
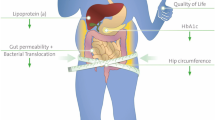
Abstract
Diabesity is showing rising prevalence. Current treatment modalities include pharmacological and non-pharmacological approaches, yet associated with various drawbacks. Recently, gut microbial dysbiosis is documented as a crucial factor in the pathogenesis of diabesity. Targeting gut microbiome using modulators shows promising therapeutic strategy for diabesity management. In this line, nanonutraceuticals represent new class of gut microbial modulators. The present article explores the potential of nanonutraceuticals including nanoprobiotics, nanoprebiotics, and plant-derived nanovesicles that are fabricated on the ecofriendly food based scaffold with gut microbial modulatory potential for diabesity management. A number of compelling evidences from different studies support Bifidobacterium, Enterococcus, and Bacteroides genera and Lactobacillus plantarum and Akkermansia muciniphila species significant in diabesity management. The probable mechanisms reported for gut microbial dysbiosis–induced diabesity are mentioned. The review findings suggest gut microbiome as significant therapeutic target for diabesity management. Moreover, ecofriendly nanonutraceuticals developed using natural products including food-grade materials are efficient modulators of gut microbiome and indicate next-generation diabesity therapeutics. Clinical studies are imperative as further exploration may provide new dimensions to the future research.
Graphical abstract



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021. https://doi.org/10.1038/s41569-020-00465-5.
Rathod P, Yadav RP. Anti-diabesity potential of various multifunctional natural molecules. J Herb Med. 2021. https://doi.org/10.1016/j.hermed.2021.100430.
Mendhe HG, Borkar SK, Shaikh M, Choudhari SG. Assessment of obesity and associated risk factors of diabesity in an urban population in Central India. Cureus. 2023. https://doi.org/10.7759/cureus.39776.
Miclotte L, Van de Wiele T. Food processing, gut microbiota and the globesity problem. Crit Rev Food Sci Nutr. 2020. https://doi.org/10.1080/10408398.2019.1596878.
Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019. https://doi.org/10.1084/jem.20180448.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022. https://doi.org/10.1136/gutjnl-2021-326789.
Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L,Savastano S et al. On behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut microbiota: a new path to treat obesity. Int J Obes Suppl. 2019; https://doi.org/10.1038/s41367-019-0011-7.
Wu J, Wang K, Wang X, Pang Y, Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021. https://doi.org/10.1007/s13238-020-00814-7.
Chawla R, Jaggi S. Medical Management of Diabesity. J Assoc Physicians India. 2019;67:52–6.
Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. The Lancet. 2022. https://doi.org/10.1016/S0140-6736(21)01919-X.
Brunton SA. Diabesity. Clin Diabetes. 2022 Fall; https://doi.org/10.2337/cd22-0088.
Leitner DR, Frühbeck G, Yumuk V, Schindler K, Micic D, Woodward E, et al. Obesity and Type 2 Diabetes: Two Diseases with a need for combined treatment strategies - EASO Can Lead the Way. Obes Facts. 2017. https://doi.org/10.1159/000480525.
Stone T, DiPietro L, Stachenfeld NS. Exercise treatment of obesity. In: Feingold KR, Anawalt B, Boyce A, et al. Editors Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [Updated 2021 May 15]. https://www.ncbi.nlm.nih.gov/books/NBK278961/. Accessed 04 July 2023.
Mutsaerts MAQ, Kuchenbecker WKH, Mol BW, Land JA, Hoek A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: a systematic review. 2013. https://doi.org/10.1093/humrep/det026.
Collins KA, Huffman KM, Wolever RQ, Smith PJ, Siegler IC, Ross LM, et al. Determinants of Dropout from and Variation in Adherence to an Exercise Intervention: The STRRIDE Randomized Trials. Transl J Am Coll Sports Med. 2022. https://doi.org/10.1249/tjx.0000000000000190.
Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep. 2017. https://doi.org/10.1007/s11892-017-0828-9.
Goswami G, Shinkazh N, Davis N. Optimal pharmacologic treatment strategies in obesity and type 2 diabetes. J Clin Med. 2014. https://doi.org/10.3390/jcm3020595.
Thibault R, Huber O, Azagury DE, Pichard C. Twelve key nutritional issues in bariatric surgery. 2016. https://doi.org/10.1016/j.clnu.2015.02.012.
Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020. https://doi.org/10.1016/j.ebiom.2019.11.051.
Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, et al. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016. https://doi.org/10.3164/jcbn.15-152.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006. https://doi.org/10.1038/nature05414.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006. https://doi.org/10.1038/4441022a.
Ghebretatios M, Schaly S, Prakash S. Nanoparticles in the food industry and their impact on human gut microbiome and diseases. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22041942.
Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021. https://doi.org/10.3390/nu14010166.
Larsen N, Vogensen FK, Van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010. https://doi.org/10.1371/journal.pone.0009085.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007. https://doi.org/10.2337/db06-1491.
Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007. https://doi.org/10.1073/pnas.0605374104.
Dahiya DK, Renuka,Puniya M,Shandilya UK, Dhewa T, Kumar N et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front Microbiol. 2017. https://doi.org/10.3389/fmicb.2017.00563.
Pedersen H, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016. https://doi.org/10.1038/nature18646.
Li X, Wang N, Yin B, Fang D, Jiang T, Fang S, et al. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol. 2016. https://doi.org/10.1111/jam.13276.
Shen Z, Zhu C, Quan Y, Yang J, Yuan W, Yang Z, et al. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J Gastroenterol Hepatol. 2018. https://doi.org/10.1111/jgh.14144.
Chang YC, Ching YH, Chiu CC, Liu JY, Hung SW, Huang WC, et al. TLR2 and interleukin-10 are involved in Bacteroides fragilis-mediated prevention of DSS-induced colitis in gnotobiotic mice. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0180025.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017. https://doi.org/10.1038/nm.4236.
Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, et al. Impact of fecal microbiota transplantation on obesity and metabolic syndrome-a systematic review. Nutrients. 2019. https://doi.org/10.3390/nu11102291.
Napolitano M, Covasa M. Microbiota transplant in the treatment of obesity and diabetes: current and future perspectives. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.590370.
Clauss M, Gérard P, Mosca A, Leclerc M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.637010.
Strasser B, Wolters M, Weyh C, Krüger K, Ticinesi A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients. 2021. https://doi.org/10.3390/nu13062045.
Allen JM, Mailing LJ, Cohrs J, Salmonson C, Fryer JD, Nehra V, et al. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes. 2018. https://doi.org/10.1080/19490976.2017.1372077.
Anderson E, Durstine JL. Physical activity, exercise, and chronic diseases: A brief review. Sports Med Health Sci. 2019. https://doi.org/10.1016/j.smhs.2019.08.006.
Rathod P, Kulkarni C, Yadav RP. Anti-diabesity principle from the seeds of Phyllanthus emblica L. Indian Drugs. 2020;57:41–50.
Upadhyay S, Yadav R, Gangan V, Kanekar Y. Compounds for treatment of lipase mediated disease. United States Patent. Patent No.: US 7355, 055 B2. 2008. Reliance Life Sciences Pvt.Ltd. Maharashtra (IN).
Santini A, Novellino E. To nutraceuticals and back: rethinking a concept. Foods. 2017. https://doi.org/10.3390/foods6090074.
Daliu P, Santini A, Novellino E. A decade of nutraceutical patents: where are we now in 2018? Expert Opin Ther Pat. 2018. https://doi.org/10.1080/13543776.2018.1552260.
AlAli M, Alqubaisy M, Aljaafari MN, AlAli AO, Baqais L, Molouki A, et al. Nutraceuticals: transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules. 2021. https://doi.org/10.3390/molecules26092540.
Shabbir U, Rubab M, Daliri EBM, Chelliah R, Javed A, Oh DH. Curcumin, quercetin, catechins and metabolic diseases: the role of gut microbiota. Nutrients. 2021. https://doi.org/10.3390/nu13010206.
Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols.Molecules (Basel, Switzerland) 2019. https://doi.org/10.3390/molecules24020370.
Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, et al. Plant-derived exosomal micro RNAs shape the gut microbiota. Cell Host Microbe. 2018. https://doi.org/10.1016/j.chom.2018.10.001.
Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020. https://doi.org/10.1038/s41467-020-18414-8.
Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, et al. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0218490.
Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017. https://doi.org/10.1016/j.molmet.2017.10.003.
Corb Aron RA, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms. 2021. https://doi.org/10.3390/microorganisms9030618.
Michael DR, Jack AA, Masetti G, Davies TS, Loxley KE, Kerry-Smith J, et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-60991-7.
Hoti G, Matencio A, Rubin Pedrazzo A, Cecone C, Appleton SL, Khazaei Monfared Y, et al. Nutraceutical concepts and dextrin-based delivery systems. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23084102.
Dima C, Assadpour E, Dima S, Jafari SM. Bioavailability of nutraceuticals: role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr Rev Food Sci Food Saf. 2020. https://doi.org/10.1111/1541-4337.12547.
Han HS, Koo SY, Choi KY. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact Mater. 2022. https://doi.org/10.1016/j.bioactmat.2021.11.027.
McClements DJ, Li F, **ao H. The nutraceutical bioavailability classification scheme: classifying nutraceuticals according to factors limiting their oral bioavailability. Annu Rev Food Sci Technol. 2015. https://doi.org/10.1146/annurev-food-032814-014043.
Durazzo A, Nazhand A, Lucarini M, Atanasov AG, Souto EB, Novellino E, et al. An updated overview on nanonutraceuticals: focus on nanoprebiotics and nanoprobiotics. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072285.
Rathod P, Yadav RP. Nanopolyphenols: perspective on oxidative stress-induced diseases. MGM J Med Sci. 2022. https://doi.org/10.4103/mgmj.mgmj_100_22.
Kučuk N, Primožič M, Knez Ž, Leitgeb M. Sustainable biodegradable biopolymer-based nanoparticles for healthcare applications. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24043188.
Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017. https://doi.org/10.2147/IJN.S127683.
Razavi S, Janfaza S, Tasnim N, Gibson DL, Hoorfar M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021. https://doi.org/10.1039/D0NA00952K.
De Souza SL, Madalena DA, Pinheiro AC, Teixeira JA, Vicente AA, Ramos ÓL, et al. Micro- and nano bio-based delivery systems for food applications: in vitro behavior. Adv Colloid Interface Sci. 2017. https://doi.org/10.1016/j.cis.2017.02.010.
Bhagit AA, Mhatre SV, Yadav RP. Proteome mediated synthesis of biocompatible green fluorescence cerium oxide quantum dots with enhanced antioxidant activity. Adv Sci Eng Med. 2020. https://doi.org/10.1166/asem.2020.2656.
Mohammadian M, Walyb MI, Moghadama M, Emam-Djomeha Z, Salamia M, Moosavi-Movahedi AA. Nanostructured food proteins as efficient systems for the encapsulation of bioactive compounds. Food Sci Hum Wellness. 2020. https://doi.org/10.1016/j.fshw.2020.04.009.
Krithika B, Preetha R. Formulation of protein based inulin incorporated synbiotic nanoemulsion for enhanced stability of probiotics. Mater Res Express. 2019;6.
Zhang F, Qiu L, Xu X, Liu Z, Zhan H, Tao X, et al. Beneficial effects of probiotic cholesterol-lowering strain of Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J Dairy Sci. 2017. https://doi.org/10.3168/jds.2016-11870.
Matsumoto Y, Ishii M, Hasegawa S, Sekimizu K. Enterococcus faecalis YM0831 suppresses sucrose-induced hyperglycemia in a silkworm model and in humans. Commun Biol. 2019. https://doi.org/10.1038/s42003-019-0407-5.
Fung WY, Yuen KH, Liong MT. Agrowaste-based nanofibers as a probiotic encapsulant: fabrication and characterization. J Agric Food Chem. 2011. https://doi.org/10.1021/jf2009342.
Ondee T, Pongpirul K, Visitchanakun P, Saisorn W, Kanacharoen S, Wongsaroj L, et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila Sci Rep. 2021. https://doi.org/10.1038/s41598-021-85449-2.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021. https://doi.org/10.1038/s41565-021-00931-2.
Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue barriers. 2016. https://doi.org/10.1080/21688370.2015.1134415.
Kim SQ, Kim KH. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: potentials in human health and disease. Cells. 2022. https://doi.org/10.3390/cells11142232.
Di Gioia S, Hossain MN, Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med (Wars). 2020. https://doi.org/10.1515/med-2020-0160.
Li A, Li D, Gu Y, Liu R, Tang X, Zhao Y, et al. Plant-derived nanovesicles: further exploration of biomedical function and application potential. APSB. 2023. https://doi.org/10.1016/j.apsb.2022.12.022.
Garaeva L, Kamyshinsky R, Kil Y, Varfolomeeva E, Verlov N, Komarova E, et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-85833-y.
Stotz HU, Brotherton D, Inal J. Communication is key: extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS Microbiol Rev. 2022. https://doi.org/10.1093/femsre/fuab044.
Liu C, Yan X, Zhang Y, Yang M, Ma Y, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 2022. https://doi.org/10.1186/s12951-022-01421-w.
Akuma P, Okagu OD, Udenigwe CC. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front Sustain Food Syst. 2019. https://doi.org/10.3389/fsufs.2019.00023.
Spinler JK, Oezguen N, Runge JK, Luna RA, Karri V, Yang J, et al. Dietary impact of a plant-derived microRNA on the gut microbiome. ExRNA. 2020. https://doi.org/10.1186/s41544-020-00053-2.
Manzaneque-López MC, Sánchez-López CM, Pérez-Bermúdez P, Soler C, Marcilla A. Dietary-derived exosome-like nanoparticles as bacterial modulators: beyond MICRORNAS. Nutrients. 2023. https://doi.org/10.3390/nu15051265.
Lei C, Teng Y, He L, Sayed M, Mu J, Xu F et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay.iScience 2021. https://doi.org/10.1016/j.isci.2021.102511.
Perut F, Roncuzzi L, Avnet S, Massa A, Zini N, Sabbadini S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021. https://doi.org/10.3390/biom11010087.
Chen Q, Li Q, Liang Y, Zu M, Chen N, Canup BSB, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022. https://doi.org/10.1016/j.apsb.2021.08.016.
Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021. https://doi.org/10.1016/j.ymthe.2020.11.030.
Ansari A, Hussain A, Wadekar R, Altamimi MA, Malik A, Mujtaba MA et al. Nanovesicles based drug targeting to control tumor growth and metastasis. Adv Cancer Biol. – Metastasis 2023; https://doi.org/10.1016/j.adcanc.2022.100083.
Paolino D, Mancuso A, Cristiano MC, Froiio F, Lammari N, Celia C, et al. Nanonutraceuticals: the new frontier of supplementary food. Nanomaterials (Basel). 2021. https://doi.org/10.3390/nano11030792.
Abdifetah O, Na-Bangchang K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: a systematic review. Int J Nanomedicine. 2019. https://doi.org/10.2147/IJN.S213229.
Song Z, Zhu W, Song J, Wei P, Yang F, Liu N, et al. Linear-dendrimer type methoxy-poly (ethylene glycol)-b-poly (ε-caprolactone) copolymer micelles for the delivery of curcumin. Drug Deliv. 2015. https://doi.org/10.3109/10717544.2014.901436.
Liu E, Zhang M, Huang Y. Pharmacokinetics and Pharmacodynamics (PK/PD) of Bionanomaterials. In: Zhao Y,Shen Y, editors. Biomed Nanomater. 2016. https://doi.org/10.1002/9783527694396.ch1.
Nunes SS, Fernandes RS, Cavalcante CH, da Costa CI, Leite EA, Lopes SCA, et al. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv Transl Res. 2019. https://doi.org/10.1007/s13346-018-0583-8.
Fan W, Peng H, Yu Z, Wang L, He H, Ma Y, et al. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm Sin B. 2022. https://doi.org/10.1016/j.apsb.2021.11.016.
Yang X, Liu Y, Zhao Y, Han M, Guo Y, Kuang H, et al. A stabilizer-free and organic solvent-free method to prepare 10-hydroxycamptothecin nanocrystals: in vitro and in vivo evaluation. Int J Nanomedicine. 2016. https://doi.org/10.2147/IJN.S102726.
Luan Q, Zhou W, Zhang H, Bao Y, Zheng M, Shi J, et al. Cellulose-based composite macrogels from cellulose fiber and cellulose nanofiber as intestine delivery vehicles for probiotics. J Agric Food Chem. 2018. https://doi.org/10.1021/acs.jafc.7b04754.
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017. https://doi.org/10.1016/j.ymthe.2017.01.025.
Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006. https://doi.org/10.2337/dc06-9912.
Park JC, Im SH. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020. https://doi.org/10.1038/s12276-020-0473-2.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005. https://doi.org/10.1073/pnas.0504978102.
Chen Z, Zhou D, Han S, Zhou S, Jia G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part Fibre Toxicol. 2019. https://doi.org/10.1186/s12989-019-0332-2.
Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett. 2018. https://doi.org/10.1186/s11671-018-2457-x.
Bellmann S, Carlander D, Fasano A, Momcilovic D, Scimeca JA, Waldman WJ, et al. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. https://doi.org/10.1002/wnan.1333.
Van den Brule S, Ambroise J, Lecloux H, Levard C, Soulas R, De Temmerman PJ, et al. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part Fibre Toxicol. 2015. https://doi.org/10.1186/s12989-016-0149-1.
Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012. https://doi.org/10.1146/annurev-bioeng-071811-150124.
Lushchak O, Zayachkivska A, Vaiserman A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: a hypothetical background and translational perspectives. Oxid Med Cell Longev. 2018. https://doi.org/10.1155/2018/3407375.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018. https://doi.org/10.1186/s12951-018-0392-8.
Acknowledgements
We thank Dr. S.N. Kadam, Former Vice Chancellor, MGM Institute of Health Sciences, Navi Mumbai, for his encouragement and support.
Author information
Authors and Affiliations
Contributions
Priyanka Rathod: conceptualization; data curation; validation; formal analysis; roles/writing, original draft; writing—review and editing. Raman P. Yadav: Conceptualization; supervision; validation; visualization; roles/writing, original draft; writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Priyanka Rathod (First author) has completed her Ph.D. degree and now she has left the organization. At the time of first submission, she was associated with the organization.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathod, P., Yadav, R.P. Gut microbiome as therapeutic target for diabesity management: opportunity for nanonutraceuticals and associated challenges. Drug Deliv. and Transl. Res. 14, 17–29 (2024). https://doi.org/10.1007/s13346-023-01404-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01404-w