Abstract
Background and Objective
In vitro glucuronidation of 17β-estradiol (estradiol) is often performed to assess the role of uridine 5′-diphospho-glucuronosyltransferase 1A1 (UGT1A1) in xenobiotic/drug metabolism. The objective of this study was to determine the effects of four commonly used organic solvents [i.e., dimethyl sulfoxide (DMSO), methanol, ethanol, and acetonitrile] on the glucuronidation kinetics of estradiol, which can be glucuronidated at C3 and C17 positions.
Methods
The impacts of organic solvents on estradiol glucuronidation were determined by using expressed UGT enzymes and liver microsomes from both human and animals.
Results
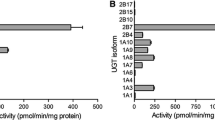
In human liver microsomes (HLM), methanol, ethanol, and acetonitrile significantly altered estradiol glucuronidation kinetics with increased Vmax (up to 2.6-fold) and CLmax (up to 2.8-fold) values. Altered estradiol glucuronidation in HLM was deduced to be attributed to the enhanced metabolic activities of UGT1A1 and UGT2B7, whose activities differ at the two glucuronidation positions. The effects of organic solvents on estradiol glucuronidation were glucuronidation position-, isozyme-, and solvent-specific. Furthermore, both ethanol and acetonitrile have a greater tendency to modify the glucuronidation activity of estradiol in animal liver microsomes.
Conclusion
Organic solvents such as methanol, ethanol, and acetonitrile showed great potential in adjusting the glucuronidation of estradiol. DMSO is the most suitable solvent due to its minimal influence on estradiol glucuronidation. Researchers should be cautious in selecting appropriate solvents to get accurate results when assessing the metabolism of a new chemical entity.








Similar content being viewed by others
References
Li YH, Meng Q, Yang MB, Liu DY, Hou XY, Tang L, et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2019;9:1113–44.
Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45:1121–32.
Meech R, Hu DG, McKinnon RA, Mubarokah SN, Haines AZ, Nair PC, et al. The Udp-glycosyltransferase (Ugt) superfamily: new members, new functions, and novel paradigms. Physiol Rev. 2019;99:1153–222.
Hu DG, Hulin JUA, Nair PC, Haines AZ, McKinnon RA, Mackenzie PI, et al. The UGTome: the expanding diversity of UDP glycosyltransferases and its impact on small molecule metabolism. Pharmacol Ther. 2019;204: 107414.
Brierley CH, Burchell B. Human UDP-glucuronosyl transferases: chemical defence, jaundice and gene therapy. BioEssays. 1993;15:749–54.
Itaaho K, Mackenzie PI, Ikushiro S, Miners JO, Finel M. The configuration of the 17-hydroxy group variably influences the glucuronidation of beta-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2008;36:2307–15.
Zhou J, Tracy TS, Remmel RP. Correlation between bilirubin glucuronidation and estradiol-3-gluronidation in the presence of model UDP-glucuronosyltransferase 1A1 substrates/inhibitors. Drug Metab Dispos. 2011;39:322–9.
Williams JA, Ring BJ, Cantrell VE, Campanale K, Jones DR, Hall SD, et al. Differential modulation of UDP-glucuronosyltransferase 1A1 (UGT1A1)-catalyzed estradiol-3-glucuronidation by the addition of UGT1A1 substrates and other compounds to human liver microsomes. Drug Metab Dispos. 2002;30:1266–73.
Fisher MB, Vandenbranden M, Findlay K, Burchell B, Thummel KE, Hall SD, et al. Tissue distribution and interindividual variation in human UDP-glucuronosyltransferase activity: relationship between UGT1A1 promoter genotype and variability in a liver bank. Pharmacogenetics. 2000;10:727–39.
Fisher MB, Campanale K, Ackermann BL, VandenBranden M, Wrighton SA. In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos. 2000;28:560–6.
Miners JO, Rowland A, Novak JJ, Lapham K, Goosen TC. Evidence-based strategies for the characterisation of human drug and chemical glucuronidation in vitro and UDP-glucuronosyltransferase reaction phenoty**. Pharmacol Ther. 2021;218:107689.
Zientek MA, Youdim K. Reaction phenoty**: advances in the experimental strategies used to characterize the contribution of drug-metabolizing enzymes. Drug Metab Dispos. 2015;43:163–81.
Easterbrook J, Lu C, Sakai Y, Li AP. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos. 2001;29:141–4.
Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, et al. Human udp-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos. 2004;32:413–23.
Dehal SS, Gagne PV, Crespi CL, Patten CJ. Effect of commom organic solvents on human UGT enzyme activities. Drug Metab Rev. 2002;34:185.
Dehal SS, Gagne PV, Crespi CL, Patten CJ. Differential effect of common organic solvents on human UGT enzyme activities. Drug Metab Rev. 2003;35:60.
Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6:140–8.
Wu BJ, Dong D, Hu M, Zhang SX. Quantitative prediction of glucuronidation in humans using the in vitro-in vivo extrapolation approach. Curr Top Med Chem. 2013;13:1343–52.
Lu DY, Ma ZG, Zhang TP, Zhang XW, Wu BJ. Metabolism of the anthelmintic drug niclosamide by cytochrome P450 enzymes and UDP-glucuronosyltransferases: metabolite elucidation and main contributions from CYP1A2 and UGT1A1. Xenobiotica. 2016;46:1–13.
Lu DY, Liu H, Ye WC, Wang Y, Wu BJ. Structure- and isoform-specific glucuronidation of six curcumin analogs. Xenobiotica. 2017;47:304–13.
Hutzler JM, Tracy TS. Atypical kinetic profiles in drug metabolism reactions. Drug Metab Dispos. 2002;30:355–62.
Soars MG, Ring BJ, Wrighton SA. The effect of incubation conditions on the enzyme kinetics of udp-glucuronosyltransferases. Drug Metab Dispos. 2003;31:762–7.
Alkharfy KM, Frye RF. Sensitive liquid chromatographic method using fluorescence detection for the determination of estradiol 3- and 17-glucuronides in rat and human liver microsomal incubations: formation kinetics. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:33–8.
Pfeiffer E, Treiling CR, Hoehle SI, Metzler M. Isoflavones modulate the glucuronidation of estradiol in human liver microsomes. Carcinogenesis. 2005;26:2172–8.
Ohno S, Naka** S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37:32–40.
Zhu L, **ao L, **a Y, Zhou K, Wang H, Huang M, et al. Diethylstilbestrol can effectively accelerate estradiol-17-O-glucuronidation, while potently inhibiting estradiol-3-O-glucuronidation. Toxicol Appl Pharmacol. 2015;283:109–16.
Zhou J, Tracy TS, Remmel RP. Glucuronidation of dihydrotestosterone and trans-androsterone by recombinant UDP-glucuronosyltransferase (UGT) 1A4: evidence for multiple UGT1A4 aglycone binding sites. Drug Metab Dispos. 2010;38:431–40.
Uchaipichat V, Galetin A, Houston JB, Mackenzie PI, Williams JA, Miners JO. Kinetic modeling of the interactions between 4-methylumbelliferone, 1-naphthol, and zidovudine glucuronidation by udp-glucuronosyltransferase 2B7 (UGT2B7) provides evidence for multiple substrate binding and effector sites. Mol Pharmacol. 2008;74:1152–62.
Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616.
Shelby MK, Cherrington NJ, Vansell NR, Klaassen CD. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos. 2003;31:326–33.
Buckley DB, Klaassen CD. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos. 2007;35:121–7.
Kuo CK, Hanioka N, Hoshikawa Y, Oguri K, Yoshimura H. Species difference of site-selective glucuronidation of morphine. J Pharmacobiodyn. 1991;14:187–93.
Xu L, Krenitsky DM, Seacat AM, Butenhoff JL, Tephly TR, Anders MW. N-Glucuronidation of perfluorooctanesulfonamide by human, rat, dog, and monkey liver microsomes and by expressed rat and human UDP-glucuronosyltransferases. Drug Metab Dispos. 2006;34:1406–10.
Liu Y, Coughtrie MW. Revisiting the latency of uridine diphosphate-glucuronosyltransferases (UGTs)—how does the endoplasmic reticulum membrane influence their function? Pharmaceutics. 2017;9:32.
Lu D, **e Q, Wu B. N-Glucuronidation catalyzed by UGT1A4 and UGT2B10 in human liver microsomes: assay optimization and substrate identification. J Pharm Biomed Anal. 2017;145:692–703.
Nirogi R, Kandikere V, Bhyrapuneni G, Ponnamaneni RK, Palacharla R, Manoharan A. Effect of dimethyl sulfoxide on in vitro cytochrome P4501A2 mediated phenacetin O-deethylation in human liver microsomes. Drug Metab Dispos. 2011;39:2162–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors Contributions
CW, ML, DL: participated in research design; CW, ML, DX, DL: conducted experiments; CW, ML, DX, SZ, JX: performed data analysis; CW, ML, DL: wrote or contributed to the writing of the manuscript; DL: Funding acquisition.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (nos. 2023A1515030140 and 2021A1515011256) and the Science and Technology Projects in Guangzhou (no. 202201011284).
Data Availability
The data are available from the corresponding author and shall be shared upon a reasonable request.
Code Availability
Not applicable.
Conflict of Interest
The authors report there are no competing interests to declare.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, C., Luo, M., **e, D. et al. Kinetic Characterization of Estradiol Glucuronidation by Liver Microsomes and Expressed UGT Enzymes: The Effects of Organic Solvents. Eur J Drug Metab Pharmacokinet 49, 343–353 (2024). https://doi.org/10.1007/s13318-024-00888-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-024-00888-2