Abstract
Background
A significant heart attack known as a myocardial infarction (MI) occurs when the blood supply to the heart is suddenly interrupted, harming the heart muscles due to a lack of oxygen. The incidence of myocardial infarction is increasing worldwide. A relationship between COVID-19 and myocardial infarction due to the recent COVID-19 pandemic has also been revealed.
Objective
We propose a biomarker and a method that can be used for the diagnosis of myocardial infarction, and an aptamer-based approach.
Results
For the diagnosis of myocardial infarction, an algorithm-based diagnosis method was developed using electrocardiogram data. A diagnosis method through biomarker detection was then developed.
Conclusion
Myocardial infarction is a disease that is difficult to diagnose based on the aspect of a single factor. For this reason, it is necessary to use a combination of various methods to diagnose myocardial infarction quickly and accurately. In addition, new materials such as aptamers must be grafted and integrated into new ways.
Purpose of Review
The incidence of myocardial infarction is increasing worldwide, and some studies are being conducted on the association between COVID-19 and myocardial infarction. The key to properly treating myocardial infarction is early detection, thus we aim to do this by offering both tools and techniques as well as the most recent diagnostic techniques.
Recent Findings
Myocardial infarction is diagnosed using an electrocardiogram and echocardiogram, which utilize cardiac signals. It is required to identify biomarkers of myocardial infarction and use biomarker-based ELISA, SPR, gold nanoparticle, and aptamer technologies in order to correctly diagnose myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a form of severe heart attack that damages the heart muscle due to a lack of oxygen due to blockage of blood flow to the heart (Li 2020). MI occurs when a coronary artery is occluded by thrombosis caused by a burst or degraded atherosclerotic plaque, resulting in myocardial necrosis (Shiffman et al. 2005). MI is caused by a sudden blockage of coronary arteries. It has a significant fatality rate. Patients may survive a heart attack. However, the majority will suffer heart failure (HF) (** et al. 2019).
Chest pain that radiates from the left arm to the neck, shortness of breath, perspiration, nausea, vomiting, irregular heartbeat, anxiety, exhaustion, weakness, stress, depression, and other causes are all signs of MI (Lu et al. 2015). For a long time, MI, one of the most frequent cardiac illnesses, has posed a severe threat to human health around the world. According to published data, the average mortality rate of MI is around 27%, making it a leading cause of death worldwide (Li 2020). High blood pressure, smoking, and obesity have all been linked to the development of MI in an epidemiological research (Jiao et al. 2018).
The coronavirus disease 2019 (COVID-19) outbreak has quickly spread over the world (Rim 2021; Toscano et al. 2021; Peltzer et al. 2022). COVID-19 has been found in recent investigations to have the capacity to impact the cardiovascular system directly or indirectly (Modin et al. 2020; Pinto and Cutlip 2020). Indeed, relevant cardiac problems in COVID-19 patients with or without preexisting cardiovascular disease have been described in various papers (Caldeira and Pinto 2021; Fardman et al. 2021; Shaw et al. 2022). The direct effects of COVID-19 infection on the cardiovascular system are more likely to result in acute myocardial infarction, heart failure, and life-threatening arrhythmias (Toscano et al. 2021). In one investigation, patients with a new diagnosis of COVID-19 had a higher risk of acute MI than noninfected controls (0.03 versus 0.01 percent; adjusted odds ratio: 1.22, 95% CI: 1.08–1.38) (Katsoularis et al. 2021).
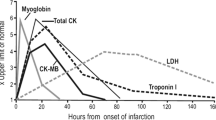
Myocardial infarction is caused by factors such as age, cigarettes, and cholesterol that affect cardiovascular disease in general (Lu et al. 2015). Cardiovascular disorders are the greatest cause of death in humans, with about 20 million people dying each year from acute cardiovascular events around the world. Every year, the global incidence of myocardial infarction rises (Wu 2021). Because of the high frequency of risk factors, 865,000 Americans are diagnosed with MI each year, with 180,000 dying from it (Shiffman et al. 2005). In addition, after the recent COVID-19 pandemic, a decrease in the number of patients with acute cardiovascular disease was observed in the early stages of COVID-19, but the mortality rate from cardiovascular causes appeared to increase (Solomon et al. 2021; Aktaa et al. 2022). Patients are hesitant to visit hospitals as a result of COVID-19. The number and death of patients with cardiovascular disease have grown after they resumed hospital care (Fox et al. 2022). The high prevalence of MI places a financial burden on both families and society. It also has an impact on the quality of life of MI patients (Wu 2021). Patients with sudden onset chest discomfort regularly visit the emergency room, yet only 15–20 percent of them have an acute myocardial infarction (Neumann et al. 2017). A patient's risk of mortality is doubled if a myocardial infarction diagnosis is missed (Graff et al. 2006). Missing a diagnosis of myocardial infarction might make the condition worse as some medications can add to the overall cardiovascular burden (Mladěnka et al. 2018). Thus, it is necessary to accurately diagnose myocardial infarction and prevent the wrong use of drugs so that they do not cause toxicity to the heart. In addition, it is necessary to diagnose myocardial infarction early and respond quickly to increase the chance of survival of patients (Aylward 1996). Since the prognosis is poor at the time of onset without specific symptoms, an efficient method for diagnosing MI is required (Fig. 1) (Bruyninckx et al. 2008).
Myocardial infarction diagnosis based on cardiac signals
Myocardial infarction symptoms are typically asymptomatic. Medical exams are often ineffective in detecting them. As a result, abnormally intense chest pain and changes in blood heart-related indicators as a result of ECG measures are used to identify it.
An electrocardiogram (ECG) is one of the most basic and quick procedures for assessing the heart. ECG plays an important role in the early diagnosis and evaluation of individuals with chest discomfort. An ECG is performed as a standard procedure for MI diagnosis because of its low cost, excellent safety, and quick reporting. Although ECG is the most common method of diagnosing acute MI, only 50–57% of patients with acute MI can be diagnosed accurately (Upasham et al. 2018; Khan et al. 2020).
To diagnose MI through ECG measurement, deep learning-based diagnostic research using electrocardiogram measurement big data of patients is also being conducted. Based on ECG results of MI patients and normal people, a deep learning algorithm for diagnosing myocardial infarction was developed and an automatic diagnosis model was developed. In the case of myocardial infarction diagnosis using deep learning, since some myocardial infarction patients have multivascular disease, an approach to a new algorithm is required to derive accurate results from these patients. In addition, there is difficulty in diagnosing MI using only ECG because NSTE-ACS patients do not show the typical ECG pattern of MI. Diagnosis of non-ST elevation acute coronary syndrome (NSTE-ACS) requires a comprehensive evaluation of changes in molecular markers such as ECG and troponin (Birnbaum and Drew 2003; Zhang et al. 2019; Cho et al. 2020; Chen 2021).
Echocardiography is a test that uses sound waves to create a real-time image of the heart. The resulting heart image is called echocardiography (Corya et al. 1975). Echocardiography is a way to monitor how the heart and valves are functioning (Mollema et al. 2009). It is tested for signs of heart problems. It is also used to diagnose cardiovascular diseases such as MI (Gibson et al. 1982). Echocardiography is an ideal method for assessing patients with MI since it is a quick, noninvasive, portable, and inexpensive imaging modality (MI). The functional result of coronary artery disease (CAD), evaluation of global and segmental wall motion, and MI consequences are all part of the echocardiographic examination (Flachskampf et al. 2011). In the case of electrocardiography and echocardiography, since diseases are diagnosed by subjective diagnosis based on patterns that appear, big data-based algorithmic approaches are increasingly used to improve accuracy. For an accurate diagnosis of myocardial infarction, it is necessary to diagnose using an objective numerical value in a way of confirming changes in biomarkers.
Molecular-based MI detection
Cardiac biomarker-based MI detection
The enzyme (EC 2.7.3.2) known as creatine kinase (CK), often referred to as creatine phosphokinase (CPK) or phosphocreatine kinase, is present in a range of tissues and cells (Aujla et al. 2019). CK catalyzes the conversion of creatine to phosphocreatine (PCr) and adenosine diphosphate (ADP) using adenosine triphosphate (ATP). Creatine kinase is measured in blood tests as a sign of CK-rich tissue damage in conditions such MI (heart attack), rhabdomyolysis (severe muscle breakdown), muscular dystrophy, autoimmune myositides, and acute kidney injury (Moghadam-Kia et al. 2016; Rashid et al. 2019). Creatin MB isoforms (CK-MB) levels can also be used to detect MI because an elevated CK-MB level is linked to myocarditis and electrical cardioversion (Wilson Tang et al. 2007).
Myoglobin (abbreviated Mb or MB) is an iron-and oxygen-binding protein found in cardiac and skeletal muscle tissues of vertebrates in general. It is practically present in all mammals. Myoglobin is only found in the bloodstream following a muscle injury in humans (Ghani et al. 2000). Myoglobin determination in combination with the detection of other biochemical markers could be particularly valuable for early triage of patients with MI (Winter et al. 2000). Because myoglobin is a sensitive marker for muscle injury, it could be used to detect a heart attack in patients who are experiencing chest pain (Weber et al. 2005). However, because increased myoglobin level has a low specificity for detecting acute myocardial infarction (AMI), other factors such as CK-MB, cardiac troponin, ECG, and clinical symptoms should be considered in the diagnosis.
Troponin, also referred to as the troponin complex, is a collection of three regulatory proteins (troponin C, troponin I, and troponin T) involved in skeletal and cardiac muscle contraction but not smooth muscle contraction (Ramachandran et al. 2013). Cardiovascular I and T troponin subtypes are sensitive and specific indications of heart muscle damage (myocardium). Blood levels of troponin are measured in patients with acute coronary syndrome or chest pain to differentiate between unstable angina and myocardial infarction (heart attack) (Molina and Segura 1984). A myocardial infarction victim will have a damaged patch of the heart muscle and elevated levels of cardiac troponin in their blood (Antman et al. 1996). Coronary vasospasm, a kind of myocardial infarction characterized by significant constriction of heart blood arteries, can also cause troponin. Troponin levels can stay elevated for up to two weeks after a myocardial infarction (January et al. 2014). Cardiac troponin is now the sole recognized biomarker that can influence a change in the care of a patient with acute coronary syndrome (Reiter et al. 2013).
Copeptin, also known as CT-proAVP, is a 39-amino-acid peptide generated from the C-terminus of arginine vasopressin pre-pro-hormone, neurophysin II, and copeptin (Nickel et al. 2012). The AVP gene encodes arginine vasopressin (AVP), also known as antidiuretic hormone (ADH). AVP is involved in many cardiovascular and renal pathways. It is linked to a variety of disorders (Mueller et al. 2018). As a result, while measuring AVP would be beneficial, it is not usually done in clinical practice due to its short half-life, which makes it difficult to quantify (Lui et al. 2015). Copeptin rapidly rises in a variety of acute conditions, including acute myocardial infarction (Keller et al. 2010). However, because copeptin is raised early in AMI and cardiac troponin (cTn) (a structural protein of cardiomyocytes) is released into the circulation in a time-dependent manner, its usage in a dual-marker method alongside cTn has a solid pathophysiological basis (Khan et al. 2007; Raskovalova et al. 2014). This method has been thoroughly tested to rule out AMI as a means of overcoming cTn release delays, particularly when less sensitive conventional cTn assays are used. Copeptin cannot be used to rule out AMI because it is increased in so many circumstances (Raskovalova et al. 2014).
Cardiac myocytes can release a tiny cytoplasmic protein (15 kDa) called heart-type Fatty Acid-Binding Protein (H-FABP) (Kleine et al. 1992; Watanabe et al. 1993). H-FABP, like other nine FABPs discovered so far, is engaged in active fatty acid metabolism, transporting fatty acids from the cell membrane to the mitochondria for oxidation (** et al. 2019). Previously, isolated rat hearts were found to emit heart-type FABP (H-FABP) at a rate or amount similar to lactate dehydrogenase after cellular damage (LDH) (Glatz et al. 1988; Ecollan et al. 2007). H-FABP is primarily found in the myocardium. It is rapidly released from the cytosol into circulation following myocardial damage (Tanaka et al. 1991). This property, along with the enhanced permeability of the endothelial barrier to small proteins, enables H-FABP to exhibit significant release early after myocardial necrosis, making it easier to detect H-FABP and providing a higher capacity for early AMI diagnosis (Li et al. 2010).
Currently, myocardial infarction is diagnosed using various biomarkers related to heart disease. Due to the recent COVID-19 pandemic, the number of patients with myocardial infarction is increasing. As a result of measuring troponin levels in COVID-19 patients in a recent study, out of 11,159 patients hospitalized for COVID-19, 6248 had troponin levels evaluated within 48 h and 4426 (71%) patients were normal. In addition, 919 (15%) had mild elevations and 902 (14%) had severe elevations of troponin (Majure et al. 2021). Moreover, patients with elevated troponin levels had an increased mortality rate than those with normal levels (Piccioni et al. 2020; Ali 2021). These levels were not associated with cardiovascular complications or elevations of inflammatory markers. Based on these results, to diagnose myocardial infarction, it is necessary to accurately diagnose MI using various markers in combination.
Predictive test after myocardial infarction diagnosis
Currently, after a diagnosis of cardiovascular disease such as myocardial infarction, major risk factors related to cardiovascular disease must be regularly measured and managed. Various parameters such as LDL cholesterol, and lipoprotein that might be caused by blood vessel obstruction in heart disease must be monitored on a regular basis.
Cholesterol is one of the factors that can affect blood vessels. Among cholesterols, LDL and HLD are representative factors (Kanter et al. 2012). The majority of cholesterol is LDL (low-density lipoprotein), also known as "bad" cholesterol. High levels of LDL cholesterol increase your risk of heart disease and stroke. The "good" cholesterol, HDL (high-density lipoprotein), absorbs cholesterol and transports it back to the liver. It is then flushed from the body by the liver. High levels of HDL cholesterol can reduce your risk of heart disease and stroke. When the body has too much LDL cholesterol, plaque can form on walls of blood vessels (Lu et al. 2015). Plaque builds up in blood vessels over time, narrowing them and blocking blood flow. Angina (chest pain) or a heart attack can be caused by obstructing blood flow to the heart. Although many patients with MI have normal HDL-C levels, studies suggest that a low blood concentration of HDL is the largest independent risk factor for CAD, resulting in an elevated risk of MI and stroke (Boden 2000). HDL tends to decrease in many patients with acute myocardial infarction. Blood HDL level below 40 mg/dL may be an effective warning signal for the development of atherosclerosis (Khan et al. 2013; Ramirez and Hu 2015).
Apolipoprotein AI (Apo-AI) is a prominent component of HDL particles. It is involved in lipid metabolism in a unique way (Frank and Marcel 2000). Apolipoprotein B is the principal apolipoprotein found in chylomicrons, VLDL, Lp(a), IDL, and LDL particles are responsible for transporting lipids, particularly cholesterol, throughout the body to all cells and organs. The primary protein component of low-density lipoprotein is apolipoprotein B (Bodde et al. 2019). LDL has varying amounts of cholesterol. However, each lipoprotein has only one protein, ApoB. Hence, ApoB is a better predictor of the number of LDL particles than LDL-C (Talmud et al. 2002; Walldius et al. 2021; Yaseen et al. 2021). In a previous study, 175,553 participants were recruited to confirm the link between apoB and apoA-I and myocardial infarction. Concentrations of apoB, apoA-I, total cholesterol, and triglycerides, the apoB/apoA-I ratio, LDL-cholesterol concentrations, and HDL-cholesterol concentrations were measured. In both men and women, apoB and apoB/apoA-I ratios were highly and positively associated with an elevated risk of fatal myocardial infarction (Walldius et al. 2001).
As it has been discovered that cardiovascular disorders such as myocardial infarction have an essential inflammatory component, C-reactive protein (CRP), an acute phase reactant as a downstream marker of inflammation, has been associated with the degree of heart damage in the initial phase of MI (Sakkinen et al. 2002; Gulhar et al. 2018). Increased concentrations of IL-6, which is produced by macrophages and adipocytes in response to a variety of acute and chronic inflammatory conditions such as bacterial, viral, or fungal infections, rheumatic and other inflammatory diseases, malignancy, and tissue injury and necrosis, can cause acute phase response (Vanhaverbeke et al. 2018). Since 2010, hsCRP (high sensitivity CRP) plasma concentration has been employed as a biomarker for disease prognosis in patients at intermediate risk for CVDs (Castro et al. 2018). In patients with a history of MI, C-reactive protein is a well-known measure of cardiovascular risk (Pagidipati et al. 2018; Lucci et al. 2020). There is mounting evidence that hs-CRP is a key indicator of cardiovascular risk. It is linked to the pathogenesis of atherosclerosis, making it useful for both primary and secondary prevention (Silva and Lacerda 2012). Therefore, hsCRP level can also be seen as a factor that requires continuous monitoring after the onset of cardiovascular diseases such as myocardial infarction.
Biomarker-based myocardial infarction diagnosis method
Many degenerative processes occur after the commencement of MI (such as the death of myocardial cells). They can progress into different diseases depending on the patient's status (Li 2020). Pathological events such as necrosis, inflammation, hemodynamic stress, and thrombosis are linked to the release of intracellular components into the bloodstream in higher concentrations than usual in cardiovascular disease. They are considered possible biomarkers (Martinez et al. 2019; Jaffe et al. 2021). In the case of myocardial infarction, its symptoms are unclear. When MI is not recognized in time, the treatment time will be missed. In general, the primary diagnosis of MI is made when pain in the arm, neck, or chest continues for more than 30 min. The diagnosis is made by checking changes in various factors, including basic examination, electrocardiogram measurement, and blood. To diagnose myocardial infarction at an early stage, studies have been actively conducted to find biomarkers that are meaningful in the diagnosis of myocardial infarction.
When myocardial infarction progressed, gene expression characteristics of a patient are analyzed to determine changes in various factors. In this study, 30,905 samples were analyzed. The MCFS method was applied for ranking analysis using the expression profile of the patient sample and the IFS method for SVM was used using the acquired feature list. Through this, 134 characteristics were selected. Factors capable of detecting myocardial infarction were selected through cluster analysis (Fig. 2). It was feasible to identify genes with elevated expression levels (DCK and RNU4-7P) and genes with decreased expression levels (KLHL8, HCLS1, MOB3A, IL17RA, ETF1, ZFAS1, CRK, MXD1, UBXN2B, FCAR and EXTL3) in post-MI by big data analysis of gene expression features. The mechanisms of MI can be revealed by analyzing changes in expression pattern (Fig. 2A). Interaction networks were revealed in some genes using gene interaction analysis of 134 distinctive genes acquired from big data analysis, and the genes IL1R1, TLR2, and TLR4 exhibited connections with MI (Fig. 2B) (Li 2020). Factors closely related to a particular disease can be identified through big data and interaction analysis, and it will be applicable to other diseases.
Heatmaps and Gene Networks for 134 Genes Chosen from all Myocardial Infarction Samples. A Gene Heat Map Results. Samples are shown in the column, while genes are in the rows. Green (D0 represents acute MI), red (D30 represents 30-days post-MI), and blue (Y1 indicates 1-year post-MI) were used to color different phases of samples. It could be shown that samples from different time points had different expression patterns. There was a comparable cluster with highly expressed genes at each time point. B These gene connections were obtained from a STRING database protein–protein interaction network and drawn using STRING's online drawing tool. Red circles indicate factors associated with myocardial infarction (Li, Ming, et al. "Identification of post-myocardial infarction blood expression signatures using multiple feature selection strategies." Frontiers in Physiology (2020): 483)
Although many studies have been conducted to detect factors that change due to the occurrence of myocardial infarction, research on materials for detecting these factors is also important. If the specificity and sensitivity of a material for diagnosis are low, even if a disease occurs, it cannot be recognized. Thus, research on the development of a material is also important.
An immunoassay using antibodies specific to the biomarker is commonly used to detect biomarkers circulating in the bloodstream (Bagyinszky et al. 2014; Ma et al. 2021). Therefore, various antibodies have been developed to detect biomarkers of myocardial infarction investigated above. An on-site diagnosis system is also being developed based on antibodies (Fathil et al. 2015).
For the diagnosis of myocardial infarction, a study was conducted on materials and methods for detecting major biomarkers that appeared in the onset of myocardial infarction. An antibody-based ELISA method was attempted using an antibody specific for biomarkers (Curley et al. 1989; Chiu et al. 1999; Panag and Goyal 2012; Rojas et al. 2018). In addition, studies have been conducted to detect biomarkers using magnetic nanoparticles (Yang et al. 2008; Seo et al. 2017), gold nanoparticles (GNP) (Sharma et al. 2018), or Surface Plasmon Resonance (SPR) technique (Wang et al. 2015; Ferreira et al. 2021) (Fig. 3). Most biomarkers for myocardial infarction are composed of proteins. Thus, antibody-based approaches are mainly used to detect biomarkers. However, a variety of methods and materials are needed to accurately diagnose and deal with myocardial infarction (Table 1).
Gold Nanoparticle-Based Myocardial Infarction Biomarker Diagnosis Methods. A Excessively high concentrations of CK-MM can complete the enzyme-substrate reaction and convert ATP to ADP, which is the initial reaction. Panel displays the mechanisms of creatine kinase sensing via an ATP-induced aggregation of initial Cys-GNP (red color) to a blue color solution, as well as an optical change in the spectra (red shift) (Sharma, Amit Kumar, et al. "Aggregation of cysteamine-capped gold nanoparticles in presence of ATP as an analytical tool for rapid detection of creatine kinase (CK-MM)." Analytica Chimica Acta 1024 (2018): 161–168). B Myoglobin detection employing hemin/G-quadruplex DNAzyme functionalized AuNPs is depicted schematically (Wang, Qing, et al. "Visual detection of myoglobin via G-quadruplex DNAzyme functionalized gold nanoparticles-based colorimetric biosensor." Sensors and Actuators B: Chemical 212 (2015): 440–445)
ELISA is a biochemical assay that uses antibodies and an enzyme-mediated color change to determine whether a sample contains antigen or antibody (Gan and Patel 2013; Chepukosi et al. 2021). The detection of very minute amounts of antigens is possible because basic immunology idea such as an antigen attaching to its specific antibody is used to diagnose diseases. In some cases, CST4 overexpression in gastrointestinal cancer was found using an ELISA test to diagnose the disease, with a sensitivity of 69 percent and a specificity of roughly 85 percent (Dou et al. 2018). Additionally, ELISA approach was used to identify and diagnose IgM/IgG produced in response to viral invasion to treat COVID-19 (Adams 2020; Tripathi and Agrawal 2020). For the diagnosis of myocardial infarction, an ELISA method grafted with various materials was tried. A dynamic and quasi-homogeneous ELISA has been reported based on the combined use of bioconjugated magnetic nanochains (MNC) and gold nanoparticle (AuNP) probes (Kumar et al. 2021; Li et al. 2021a).
Surface plasmon resonance (SPR) is a technique for detecting resonant oscillations of conduction electrons at the interface of negative and positive permittivity materials. Such oscillations are induced by incident light. The adsorption of a substance on a gold, silver, or metal nanoparticle surface is often measured. Based on the SPR technique, it was applied to detect the biomarker CD133 for diagnosing leukemia patients (Fathi et al. 1863). Additionally, SPR was used to detect food allergies using antigenic compounds found in milk, peanuts, eggs, etc. (Zhou et al. 2019). For the diagnosis of myocardial infarction biomarkers, research has been conducted by grafting them onto biomarkers such as troponin and CK (Dutra and Kubota 2007; Fathil et al. 2015; Pawula et al. 2016; Ferreira et al. 2021) (Fig. 4).
SPR-based myocardial infarction biomarker diagnosis method. A A sandwich assay SPR immunosensor with gold nanoparticles attached to a detection antibody for signal amplification is shown schematically. Sensorgram of binding experiments on active surfaces utilizing AuNP-modified detector antibodies. B Amplification of the final binding response employing detection antibody linked AuNPs for the measurement of cTnT in human serum. C On the active sensor surface, 0.5 ng\mL antigen was administered (Pawula, Maria, Zeynep Altintas, and Ibtisam E. Tothill. "SPR detection of cardiac troponin T for acute myocardial infarction." Talanta 146 (2016): 823–830)
Aptamers are DNA and RNA oligonucleotide compounds (high binding affinity) that can build loop-like three-dimensional structures to detect various structural properties of target substances (Lee et al. 2015a; Sekhon et al. 2017; Sekhon et al. 2019). They are also called ‘Chemical antibody’ due to their characteristic of specifically binding to a target substance. Aptamers are screened using SELEX (systematic evolution of ligands by exponential enrichment). Unbound substances are repeatedly removed (10–15 times) to generate substances with the highest binding affinity to the target substance (Song et al. 2017; Li et al. 2021b). Aptamers have broad target selectivity (proteins, ions, chemicals, heavy metals and cells) (Shin et al. 2020, 2022). They are inexpensive and simple to manufacture (Shin et al. 2018a; Sekhon et al. 2021). In addition, an aptamer is composed of nucleic acids such as DNA and RNA, making it easy to modify. Therefore, it can be applied as an aptamer-based sandwich assay platform. It has a feature that can be applied as a strip sensor for real-time detection (Lee et al. 2015b; Shin et al. 2018b). An aptamer has been used to create a point-of-care (POC) diagnostic sensor that can find cortisol in saliva. The aptamer-based diagnostic sensor has a detection limit of 0.37 ng/mL and can detect cortisol concentrations between 0.5 and 15 ng/mL (Dalirirad et al. 2020). Additionally, an aptamer that binds to the spike trimer antigen was chosen and employed for diagnosis to identify SARS-COV-2. This aptamer can detect an antigen at a concentration of at least 2 nM. Sensitivity and specificity for infected and uninfected people were 91 and 98 percent, respectively (Gupta et al. 2021).
As a result, an aptamer for troponin I, one of the biomarkers of myocardial infarction, has been developed lately (Zhang et al. 2020). Without the need of labeling, pre-concentration, or amplification stages, the developed aptamer can detect TnI in the range of 0.03–2.0 ng mL−1. When 89 human samples were used to test TnI detection performance of aptamers, diagnostic sensitivity and specificity were 100 and 81%, respectively (Negahdary et al. 2018). In addition, one study was done to choose an aptamer specific to Troponin among myocardial infarction biomarkers and to build a diagnostic sensor to which it was attached for the diagnosis of myocardial infarction (Jo et al. 2015; Grabowska et al. 2018) (Fig. 5).
Aptamer-based Myocardial Infarction Biomarker Diagnosis Method. A The detection of cTnI is depicted in this diagram. cTnI is introduced after cTnI aptamer is immobilized on the surface of the gold working electrode. The drop in SWV signal was used to determine the concentration of cTnI. (Jo, Hunho, et al. "Electrochemical aptasensor of cardiac troponin I for the early diagnosis of acute myocardial infarction." Analytical chemistry 87.19 (2015): 9869–9875.) B Integration of aptamers to N3-modified DNA aptamers (III) utilizing Cu(I)-catalyzed click chemistry and (IV) passivation using synthetic pyrene-PEG (green layer) (Grabowska, Iwona, et al. "Electrochemical aptamer-based biosensors for the detection of cardiac biomarkers." ACS omega 3.9 (2018): 12,010–12,018)
Conclusion
With the recent outbreak of the COVID-19 pandemic, the mortality rate due to myocardial infarction is increasing due to a decrease in the frequency of hospital visits. The incidence of myocardial infarction is also increasing in COVID-19 patients (Rattka et al. 2021). Myocardial infarction is caused by various factors such as diet and living environment (Lu et al. 2015). Blood vessels are clogged by various factors. Thus, blood flowing into the heart decreases (Muse et al. 2017). As there are no clear symptoms, factors and methods for diagnosing and monitoring myocardial infarction are required (Bruyninckx et al. 2008).
In this review, various biomarker factors and diagnostic methods for diagnosing myocardial infarction were summarized. Here, we investigated biomarker factors that could change when myocardial infarction occurred. We also summarized biomarkers used for diagnosis and methods that could derive new biomarkers. In addition, we investigated biomarker factors that could be monitored to prevent recurrence after a diagnosis of myocardial infarction. Among diagnostic methods of myocardial infarction, electrocardiogram measurement and diagnostic methods using biomarkers were investigated.
Currently, due to the development of big data and algorithms, an algorithm-based analysis method using electrocardiogram data of myocardial infarction patients is being performed to diagnose myocardial infarction (Zhang et al. 2019; Cho et al. 2020; Chen 2021). In addition, as a biomarker-based molecular diagnostic method, antibodies and aptamers against factors were developed using biomarkers of myocardial infarction (such as Creatine kinase, myoglobin, Troponin, Copeptin, and H-FABP), ELISA, strip sensor, and nanoparticle using the same (Delanghe et al. 1990; Zhu et al. 2007; Li et al. 2021a). Research has been conducted to diagnose myocardial infarction by grafting with other techniques.
Myocardial infarction is a disease that needs to be diagnosed early and dealt with quickly (Lu et al. 2015). To increase the accuracy of diagnosis, the sensitivity and specificity of detection technology must be improved. Using a range of chemicals and technologies like ELISA, SPR, and Aptamer that are used to detect diseases in a sophisticated manner, it is vital to reliably and quickly identify myocardial infarction. To prevent myocardial infarction and improve diagnosis, a technique for removing factors involved in blocking blood vessels is required.
Change history
15 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13273-022-00325-y
Abbreviations
- MI:
-
Myocardial infarction
- HF:
-
Heart failure
- LDL:
-
Low-density lipoprotein
- ECG:
-
Electrocardiogram
- NSTE-ACS:
-
Non-ST elevation acute coronary syndrome
- CAD:
-
Coronary artery disease
- CK:
-
Creatine kinase
- CPK:
-
Creatine phosphokinase
- PCr:
-
Phosphocreatine
- ADP:
-
Adenosine diphosphate
- ATP:
-
Adenosine triphosphate
- CK-MB:
-
Creatin MB isoforms
- Mb(MB):
-
Myoglobin
- AMI:
-
Acute myocardial infarction
- AVP:
-
Arginine vasopressin
- ADH:
-
Antidiuretic hormone
- cTn:
-
Cardiac troponin
- H-FABP:
-
Heart-type Fatty Acid-Binding Protein
- HDL:
-
High-density lipoprotein
- Apo-AI:
-
Apolipoprotein AI
- CRP:
-
C-reactive protein
- hsCRP:
-
High sensitivity CRP
- ELISA:
-
Enzyme-linked immunosorbent assay
- GNP(AuNP):
-
Gold nanoparticles
- SPR:
-
Surface Plasmon Resonance
- MNC:
-
Magnetic nanochains
References
Adams ER et al (2020) Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.15927.1
Aktaa S et al (2022) Quality of acute myocardial infarction care in England and Wales during the COVID-19 pandemic: linked nationwide cohort study. BMJ Qual Saf 31:116–122
Ali J et al (2021) Cardiac troponin levels in hospitalized covid-19 patients as a predictor of severity and outcome a retrospective cohort study. Cureus. https://doi.org/10.7759/cureus.14061
Antman EM et al (1996) Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 335:1342–1349
Aujla RS, Patel R (2019) Creatine phosphokinase. StatPearls, Treasure Island (FL)
Aylward P (1996) Acute myocardial infarction: early treatment. Aust Prescr 16(2):52–54
Bagyinszky E, Youn YC, An SSA, Kim S (2014) Diagnostic methods and biomarkers for Alzheimer’s disease. Toxicol Environ Heal Sci 6:133–147
Birnbaum Y, Drew BJ (2003) The electrocardiogram in ST elevation acute myocardial infarction: correlation with coronary anatomy and prognosis. Postgrad Med J 79:490–504
Bodde MC et al (2019) Apolipoproteins A1, B, and apoB/apoA1 ratio are associated with first ST-segment elevation myocardial infarction but not with recurrent events during long-term follow-up. Clin Res Cardiol 108:520–538
Boden WE (2000) High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans affairs high-density lipoprotein intervention trial. Am J Cardiol 86:19–22
Bruyninckx R, Aertgeerts B, Bruyninckx P, Buntinx F (2008) Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract 58:e1–e8
Caldeira D, Pinto FJ (2021) COVID-19 and myocardial infarction. The Lancet 398:1963–1964
Castro AR, Silva SO, Soares SC (2018) The use of high sensitivity C-reactive protein in cardiovascular disease detection. J Pharm Pharm Sci 21:496–503
Chen X et al (2021) Acute myocardial infarction detection using deep learning-enabled electrocardiograms. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2021.654515
Chepukosi KW, Nyariki JN, Jillani NE, Okanya PW, Isaac AO (2021) Manganese exacerbated chronic khat-induced neurological deficits, inflammation and organ toxicity in a mouse model. Toxicol Environ Heal Sci 13:337–350
Chiu A, Chan W-K, Cheng S-H, Leung C-K, Choi C-H (1999) Troponin-I, myoglobin, and mass concentration of creatine kinase-MB in acute myocardial infarction. QJM 92:711–718
Cho Y et al (2020) Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci Rep 10:1–10
Corya BC, Rasmussen S, Knoebel SB, Feigenbaum H (1975) Echocardiography in acute myocardial infarction. Am J Cardiol 36:1–10
Curley P, Abbott R, Vallance D (1989) Clinical application of a new enzyme-linked assay for the estimation of brain-specific creatine kinase in head injured patients. Br J Neurosurg 3:655–658
Dalirirad S, Han D, Steckl AJ (2020) Aptamer-based lateral flow biosensor for rapid detection of salivary cortisol. ACS Omega 5:32890–32898
Das UN (2016) Heart-type fatty acid-binding protein (H-FABP) and coronary heart disease. Indian Heart J 68:16
de Winter RJ, Lijmer JG, Koster RW, Hoek FJ, Sanders GT (2000) Diagnostic accuracy of myoglobin concentration for the early diagnosis of acute myocardial infarction. Ann Emerg Med 35:113–120
Delanghe J, Chapelle J-P, Vanderschueren S (1990) Quantitative nephelometric assay for determining myoglobin evaluated. Clin Chem 36:1675–1678
Dellas C et al (2014) A novel H-FABP assay and a fast prognostic score for risk assessment of normotensive pulmonary embolism. Thromb Haemost 112:996–1003
Dou Y et al (2018) Antibody-sandwich ELISA analysis of a novel blood biomarker of CST4 in gastrointestinal cancers. Onco Targets Ther 11:1743
Dutra RF, Kubota LT (2007) An SPR immunosensor for human cardiac troponin T using specific binding avidin to biotin at carboxymethyldextran-modified gold chip. Clin Chim Acta 376:114–120
Ecollan P et al (2007) Pre-hospital detection of acute myocardial infarction with ultra-rapid human fatty acid-binding protein (H-FABP) immunoassay. Int J Cardiol 119:349–354
Fardman A et al (2021) Acute myocardial infarction in the Covid-19 era: Incidence, clinical characteristics and in-hospital outcomes—a multicenter registry. PLoS ONE 16:e0253524
Fathi F, Rahbarghazi R, Movassaghpour AA, Rashidi M-R (2019) Detection of CD133-marked cancer stem cells by surface plasmon resonance: its application in leukemia patients. Biochimica Et Biophysica Acta (BBA)-Gen Subj 1863:1575–1582
Fathil M et al (2015) Diagnostics on acute myocardial infarction: cardiac troponin biomarkers. Biosens Bioelectron 70:209–220
Ferreira AL et al (2021) Development of a novel biosensor for creatine kinase (CK-MB) using surface plasmon resonance (SPR). Appl Surf Sci 554:149565
Flachskampf FA et al (2011) Cardiac imaging after myocardial infarction. Eur Heart J 32:272–283
Fox DK et al (2022) Impact of the COVID-19 pandemic on patients without COVID-19 with acute myocardial infarction and heart failure. J Am Heart Assoc 11:e022625
Frank PG, Marcel YL (2000) Apolipoprotein AI: structure–function relationships. J Lipid Res 41:853–872
Gan SD, Patel KR (2013) Enzyme immunoassay and enzyme-linked immunosorbent assay. J Invest Dermatol 133:e12
Geng T, Song Z, Zhang J, Xu Z (2017) Creatine kinase determination based on an electrochemical impedance immunosensor. Int J Electrochem Sci 12:8552–8563
Ghani F et al (2000) Role of heart-type fatty acid-binding protein in early detection of acute myocardial infarction. Clin Chem 46:718–719
Gibson RS et al (1982) Value of early two dimensional echocardiography in patients with acute myocardial infarction. Am J Cardiol 49:1110–1119
Glatz J et al (1988) Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochimica Et Biophysica Acta (BBA)-Lipids Lipid Metab 961:148–152
Grabowska I et al (2018) Electrochemical aptamer-based biosensors for the detection of cardiac biomarkers. ACS Omega 3:12010–12018
Graff LG et al (2006) Delay in the diagnosis of acute myocardial infarction: effect on quality of care and its assessment. Acad Emerg Med 13:931–938
Gulhar R, Ashraf MA, Jialal I (2018) Physiology, acute phase reactants. StatPearls, Treasure Island (FL)
Gupta A et al (2021) A novel G-quadruplex aptamer-based spike trimeric antigen test for the detection of SARS-CoV-2. Mol Ther-Nucleic Acids 26:321–332
Haque A-MJ, Kim J, Dutta G, Kim S, Yang H (2015) Redox cycling-amplified enzymatic Ag deposition and its application in the highly sensitive detection of creatine kinase-MB. Chem Commun 51:14493–14496
Jaffe AS et al (2021) ESC study group on cardiac biomarkers of the association for acute cardiovascular care: a fond farewell at the retirement of CKMB. Eur Heart J 42(23):2260–2264
January CT et al (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 64:2246–2280
Jiao N, Qi Y, Lv C, Li H, Yang F (2018) Identification of protein complexes associated with myocardial infarction using a bioinformatics approach. Mol Med Rep 18:3569–3576
** D et al (2019) A chymase inhibitory RNA aptamer improves cardiac function and survival after myocardial infarction. Mol Ther-Nucleic Acids 14:41–51
Jo H et al (2015) Electrochemical aptasensor of cardiac troponin I for the early diagnosis of acute myocardial infarction. Anal Chem 87:9869–9875
Kanter MM, Kris-Etherton PM, Fernandez ML, Vickers KC, Katz DL (2012) Exploring the factors that affect blood cholesterol and heart disease risk: is dietary cholesterol as bad for you as history leads us to believe? Adv Nutr 3:711–717
Kato K et al (1985) Highly sensitive enzyme immunoassay for human creatine kinase BB isozyme. Clin Chim Acta 150:31–40
Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Connolly A-MF (2021) Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet 398:599–607
Keller T et al (2010) Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 55:2096–2106
Khan SQ et al (2007) C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester acute myocardial infarction peptide (LAMP) study. Circulation 115:2103–2110
Khan HA, Alhomida AS, Sobki SH (2013) Lipid profile of patients with acute myocardial infarction and its correlation with systemic inflammation. Biomark Insights 8:BMI-S11015
Khan S et al (2020) Gold nanoparticle-based platforms for diagnosis and treatment of myocardial infarction. ACS Biomater Sci Eng 6:6460–6477
Kleine AH, Glatz JF, Nieuwenhoven FAV, Vusse GJ (1992) Lipid metabolism in the healthy and disease heart. Springer, US, pp 155–162
Kumar N, Verma KL, Jain VK, Nagpal S (2021) Integrated device for colorimetric detection of arsenite using polyethylene glycol capped gold nanoparticles—lab-on-chip. Toxicol Environ Heal Sci 13:351–362
Lee S-H et al (2015a) Analytical bioconjugates, aptamers, enable specific quantitative detection of Listeria monocytogenes. Biosens Bioelectron 68:272–280
Lee K-A et al (2015b) Aptamer-based sandwich assay and its clinical outlooks for detecting lipocalin-2 in hepatocellular carcinoma (HCC). Sci Rep 5:1–13
Li C-J et al (2010) Point-of-care test of heart-type fatty acid-binding protein for the diagnosis of early acute myocardial infarction. Acta Pharmacol Sin 31:307–312
Li M et al (2020) Identification of post-myocardial infarction blood expression signatures using multiple feature selection strategies. Fron Physiol. https://doi.org/10.3389/fphys.2020.00483
Li D et al (2021a) Magnetic nanochains-based dynamic ELISA for rapid and ultrasensitive detection of acute myocardial infarction biomarkers. Anal Chim Acta 1166:338567
Li L et al (2021b) Nucleic acid aptamers for molecular diagnostics and therapeutics: advances and perspectives. Angew Chem Int Ed 60:2221–2231
Lu L, Liu M, Sun R, Zheng Y, Zhang P (2015) Myocardial infarction: symptoms and treatments. Cell Biochem Biophys 72:865–867
Lucci C et al (2020) Prognostic impact of admission high-sensitivity C-reactive protein in acute myocardial infarction patients with and without diabetes mellitus. Cardiovasc Diabetol 19:1–13
Lui CT et al (2015) Role of copeptin in dual–cardiac marker strategy for patients with chest pain presented to ED. Am J Emerg Med 33:1732–1736
Ma H, Cassedy A, O’Kennedy R (2021) The role of antibody-based troponin detection in cardiovascular disease: A critical assessment. J Immunol Methods 497:113108
Majure DT et al (2021) Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Am J Cardiol 138:100–106
Martinez PF, Oliveira-Junior SA, Polegato BF, Okoshi K, Okoshi MP (2019) Biomarkers in acute myocardial infarction diagnosis and prognosis. Arq Bras Cardiol 113(1):40–41
Mladěnka P et al (2018) Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev 38:1332–1403
Modin D et al (2020) Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 142:2080–2082
Moghadam-Kia S, Oddis CV, Aggarwal R (2016) Approach to asymptomatic creatine kinase elevation. Clevel Clin J Med 83:37
Molina E, Segura V. Anales de Medicina Interna (Madrid, Spain: 1984). pp. 283–288.
Mollema SA, Nucifora G, Bax JJ (2009) Prognostic value of echocardiography after acute myocardial infarction. Heart 95:1732–1745
Moreira FT, Dutra RA, Noronha JP, Sales MGF (2014) Novel sensory surface for creatine kinase electrochemical detection. Biosens Bioelectron 56:217–222
Mueller C et al (2018) Use of copeptin for rapid rule-out of acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 7:570–576
Muse ED et al (2017) A whole blood molecular signature for acute myocardial infarction. Sci Rep 7:1–9
Negahdary M, Behjati-Ardakani M, Sattarahmady N, Heli H (2018) An aptamer-based biosensor for troponin I detection in diagnosis of myocardial infarction. J Biomed Phys Eng 8:167
Neumann JT et al (2017) Early diagnosis of acute myocardial infarction using high-sensitivity troponin I. PLoS ONE 12:e0174288
Nickel CH, Bingisser R, Morgenthaler NG (2012) The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med 10:1–6
Pagidipati NJ et al (2018) High-sensitivity C-reactive protein elevation in patients with prior myocardial infarction in the United States. Am Heart J 204:151–155
Panag, K. & Goyal, S. Evaluation of Creatine Kinase as a Diagnostic Tool for Thyroid Function. (2012).
Pawula M, Altintas Z, Tothill IE (2016) SPR detection of cardiac troponin T for acute myocardial infarction. Talanta 146:823–830
Peltzer PM et al (2022) Risk of chlorine dioxide as emerging contaminant during SARS-CoV-2 pandemic: enzyme, cardiac, and behavior effects on amphibian tadpoles. Toxicol Environ Heal Sci 14:47–57
Piccioni A et al (2020) Role of troponin in COVID-19 pandemic: a review of literature. Eur Rev Med Pharmacol Sci 24:10293–10300
Pinto D, Cutlip D (2020) COVID-19: myocardial infarction and other coronary artery disease issues. Uptodate. Searchbox. Science
Ramachandran G et al (2013) An in silico analysis of troponin I mutations in hypertrophic cardiomyopathy of Indian origin. PLoS ONE 8:e70704
Ramirez A, Hu P (2015) Low high-density lipoprotein and risk of myocardial infarction. Clin Med Insights: Cardiol 9:CMC-S26624
Rashid S, Malik A, Khurshid R, Faryal U, Qazi S (2019) The diagnostic value of biochemical cardiac markers in acute myocardial infarction. Myocardial infarction. Intechopen, UK. https://doi.org/10.5772/intechopen.76150
Raskovalova T et al (2014) Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 3:18–27
Rattka M et al (2021) Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart 107:482–487
Reiter M et al (2013) Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction. Heart 99:708–714
Rim K-T (2021) COVID-19 pandemic and the protection of workers’ health from disinfectant chemicals. Toxicol Environ Heal Sci 13:1–7
Rojas V et al (2018) Detection of muscle-specific creatine kinase expression as physiological indicator for Atlantic salmon (Salmo salar L) skeletal muscle damage. Aquaculture 496:66–72
Sakkinen P et al (2002) C-reactive protein and myocardial infarction. J Clin Epidemiol 55:445–451
Sekhon SS et al (2017) Defining the copper binding aptamotif and aptamer integrated recovery platform (AIRP). Nanoscale 9:2883–2894
Sekhon SS et al (2019) The Role of aptamer loaded exosome complexes in the neurodegenerative diseases. Toxicol Environ Heal Sci 11:85–93
Sekhon SS, Kaur P, Kim Y-H, Sekhon SS (2021) 2D graphene oxide–aptamer conjugate materials for cancer diagnosis. Npj 2D Mater Appl 5:1–19
Seo DY, ** M, Ryu J-C, Kim Y-J (2017) Investigation of the genetic toxicity by dextran-coated superparamagnetic iron oxide nanoparticles (SPION) in HepG2 cells using the comet assay and cytokinesis-block micronucleus assay. Toxicol Environ Heal Sci 9:23–29
Sharma AK, Pandey S, Nerthigan Y, Swaminathan N, Wu H-F (2018) Aggregation of cysteamine-capped gold nanoparticles in presence of ATP as an analytical tool for rapid detection of creatine kinase (CK-MM). Anal Chim Acta 1024:161–168
Shaw P et al (2022) COVID-19 outcomes in patients hospitalised with acute myocardial infarction (AMI): a protocol for systematic review and meta-analysis. COVID 2:138–147
Shiffman D et al (2005) Identification of four gene variants associated with myocardial infarction. Am J Hum Genet 77:596–605
Shin W-R et al (2018a) Aptamer-based paper strip sensor for detecting Vibrio fischeri. ACS Comb Sci 20:261–268
Shin W-R et al (2018b) Aptamer-based pathogen monitoring for Salmonella enterica ser Typhimurium. J Biomed Nanotechnol 14:1992–2002
Shin W-R et al (2020) Aptamer-linked immobilized sorbent assay for detecting GMO marker, phosphinothricin acetyltransferase (PAT). Mol Cell Toxicol 16:253–261
Shin W-R et al (2022) Structure based innovative approach to analyze aptaprobe–GPC3 complexes in hepatocellular carcinoma. J Nanobiotechnol 20:1–17
Shu-Hai J et al (2014) The detection of CTN I by the aptamer biosensor. Prog Biochem Biophys 41:916–920
Silva D, de Lacerda AP (2012) High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol 31:733–745 (English Edition)
Solomon MD et al (2021) Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID-19 surges. JAMA 326:82–84
Song M-S et al (2017) Detecting and discriminating Shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules 22:825
Talmud PJ, Hawe E, Miller GJ, Humphries SE (2002) Nonfasting apolipoprotein B and triglyceride levels as a useful predictor of coronary heart disease risk in middle-aged UK men. Arterioscler Thromb Vasc Biol 22:1918–1923
Tanaka T, Hirota Y, Sohmiya K-I, Nishimura S, Kawamura K (1991) Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem 24:195–201
Toscano O, Cosentino N, Campodonico J, Bartorelli AL, Marenzi G (2021) Acute myocardial infarction during the COVID-19 pandemic: an update on clinical characteristics and outcomes. Front Cardiovasc Med 8:648290
Tripathi S, Agrawal A (2020) Blood plasma microfluidic device: aiming for the detection of COVID-19 antibodies using an on-chip ELISA platform. Trans Indian Natl Acad Eng 5:217–220
Upasham S, Tanak A, Prasad S (2018) Cardiac troponin biosensors: where are we now. Adv Heal Care Technol 4:1–13
Vanhaverbeke M et al (2018) C-reactive protein during and after myocardial infarction in relation to cardiac injury and left ventricular function at follow-up. Clin Cardiol 41:1201–1206
Walldius G et al (2001) High apolipoprotein B, low apolipoprotein AI, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. The Lancet 358:2026–2033
Walldius G et al (2021) Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio—experience from the Swedish AMORIS cohort: a cohort study. PLoS Med 18:e1003853
Wang Q, Yang X, Yang X, Liu F, Wang K (2015) Visual detection of myoglobin via G-quadruplex DNAzyme functionalized gold nanoparticles-based colorimetric biosensor. Sens Actuators, B Chem 212:440–445
Wang Y et al (2019) A sensitive and rapid chemiluminescence immunoassay for point-of-care testing (POCT) of copeptin in serum based on high-affinity monoclonal antibodies via cytokine-assisted immunization. Int J Nanomed 14:4293
Watanabe K, Wakabayashi H, Veerkamp J, Ono T, Suzuki T (1993) Immunohistochemical distribution of heart-type fatty acid-binding protein immunoreactivity in normal human tissues and in acute myocardial infarct. J Pathol 170:59–65
Weber M et al (2005) Diagnostic utility of new immunoassays for the cardiac markers cTnI, myoglobin and CK-MB mass. Clin Biochem 38:1027–1030
Wei J et al (2003) A novel sandwich immunosensing method for measuring cardiac troponin I in sera. Anal Biochem 321:209–216
Wilson Tang W et al (2007) National academy of clinical biochemistry laboratory medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation 116:e99–e109
Wu Y et al (2021) Diagnostic and prognostic biomarkers for myocardial infarction. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2020.617277
Yang S-Y et al (2008) Dual immobilization and magnetic manipulation of magnetic nanoparticles. J Magn Magn Mater 320:2688–2691
Yaseen RI, El-Leboudy MH, El-Deeb HM (2021) The relation between ApoB/ApoA-1 ratio and the severity of coronary artery disease in patients with acute coronary syndrome. Egypt Heart J 73:1–9
Zhang N et al (2019) Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology 291:606–617
Zhang J, Lakshmipriya T, Gopinath SC (2020) Electroanalysis on an interdigitated electrode for high-affinity cardiac troponin I biomarker detection by aptamer-gold conjugates. ACS Omega 5:25899–25905
Zhou J et al (2019) Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens Bioelectron 142:111449
Zhu B-L et al (2007) Postmortem cardiac troponin I and creatine kinase MB levels in the blood and pericardial fluid as markers of myocardial damage in medicolegal autopsy. Leg Med 9:241–250
Zhu X-D et al (2011) Detection of copeptin in peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Crit Care 15:1–13
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2021R1F1A1063744) and Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2020R1A6A1A06046235), Republic of Korea.
Funding
NRF, No.2021R1F1A1063744, Yang-Hoon Kim.
Author information
Authors and Affiliations
Contributions
J-PL and SYK designed the study. J-PL and W-RS wrote the paper. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Sang Young Kim declares that he has no conflict of interest. **-Pyo Lee declares that he has no conflict of interest. Woo-Ri Shin declares that she has no conflict of interest. In-Hwan Oh declares that he has no conflict of interest. Ji-Young Ahn declares that she has no conflict of interest. Yang-Hoon Kim declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The original online version of this article was revised: In this article an article note has been added and affiliation 2 updated.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S.Y., Lee, JP., Shin, WR. et al. Cardiac biomarkers and detection methods for myocardial infarction. Mol. Cell. Toxicol. 18, 443–455 (2022). https://doi.org/10.1007/s13273-022-00287-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-022-00287-1