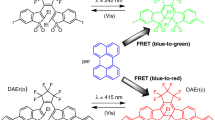
Abstract
Fluorescent conjugated polymers (CPs) emitting blue, green, and red were polymerized via the Suzuki coupling reaction. Their emissive monomer units were linked to a fluorene unit in the backbone and thus could be excited at the same wavelength to fluoresce their own emission colors. The resultant polymers were dissolved in dioctyl phthalate, which was embedded in melamine–formaldehyde microcapsules (MFMs). The CP-embedded MFMs maintained the red, green, and blue emission colors of the CPs. The three fluorescent MFMs were mixed in suitable amounts to fabricate white light-emitting MFMs under single-wavelength excitation. The microcapsule embedment could effectively avoid the unnecessary energy transfer from short- to long-wavelength emission, which is a crucial obstacle for white emission when using mixed colors. Thus, white light emission was successfully obtained using spatially separated MFMs in aqueous solution.
Graphical Abstract
White light emission was performed using spatially separated RGB conjugated polymers embedded in melamine-formaldehyde microcapsules.








Similar content being viewed by others
References
T. Wang, K. Li, B. Yao, Y. Chen, H. Zhan, Z. **e, G. **e, X. Yi, Y. Cheng, Adv. Funct. Mater. 30, 2002493 (2020)
L.H. **e, C.R. Yin, W.Y. Lai, Q.L. Fan, W. Huang, Prog. Polym. Sci. 37, 1192 (2012)
G.M. Farinola, R. Ragni, Chem. Soc. Rev. 40, 3467 (2011)
Y. He, X. Hu, Z. Gong, S. Chen, R. Yuan, Mikrochim. Acta 187, 237 (2020)
J.H. Luo, Q. Li, S.H. Chen, R. Yuan, A.C.S. Appl, Mater. Interfaces 11, 27363 (2019)
W. Dong, Z. Ma, P. Chen, Q. Duan, Mater. Lett. 236, 480 (2019)
M. Shang, C. Li, J. Lin, Chem. Soc. Rev. 43, 1372 (2014)
K. Chang, X. Men, H. Chen, Z. Liu, S. Yin, W. Qin, Z. Yuan, C. Wu, J. Mater. Chem. C 3, 7281 (2015)
S. Jo, J. Kim, J. Noh, D. Kim, G. Jang, N. Lee, E. Lee, T.S. Lee, A.C.S. Appl, Mater. Interfaces 6, 22884 (2014)
J. Kim, T.S. Lee, Macromol. Rapid Commun. 37, 303 (2016)
M. Huang, R. Yu, K. Xu, S. Ye, S. Kuang, X. Zhu, Y. Wan, Chem. Sci. 7, 4485 (2016)
Y. Huang, J. **ng, Q. Gong, L.C. Chen, G. Liu, C. Yao, Z. Wang, H.L. Zhang, Z. Chen, Q. Zhang, Nat. Commun. 10, 169 (2019)
B.-P. Jiang, Y.-X. Yu, X.-L. Guo, Z.-Y. Ding, B. Zhou, H. Liang, X.-C. Shen, Carbon 128, 12 (2018)
G. Feng, B. Liu, Acc. Chem. Res. 51, 1404 (2018)
F. Wurthner, Angew. Chem. Int. Ed. 59, 14192 (2020)
H. Wang, G. Liu, J. Mat. Chem. B 6, 4029 (2018)
B. Kumari, R. Dahiwadkar, S. Kanvah, Aggregate. 3, 191 (2022)
F. Zhang, H. **e, B. Guo, C. Zhu, J. Xu, Polym. Chem. 13, 8 (2022)
C. Zhu, R.T.K. Kwok, J.W.Y. Lam, B.Z. Tang, A.C.S. Appl, Bio Mater. 1, 1768 (2018)
C. Liu, J.-C. Yang, J.W.Y. Lam, H.-T. Feng, B.Z. Tang, Chem. Sci. 13, 611 (2022)
J. Li, J. Wang, H. Li, N. Song, D. Wang, B.Z. Tang, Chem. Soc. Rev. 49, 1144 (2020)
Y. Gwon, S. Jo, H.-J. Lee, S.Y. Park, T.S. Lee, Polymer 229, 124004 (2021)
S. Jo, H. Kim, T.S. Lee, Polymer 228, 123892 (2021)
S. Jo, D. Kim, S.-H. Son, Y. Kim, T.S. Lee, A.C.S. Appl, Mater. Interfaces 6, 1330 (2014)
D. Kim, J. Kim, T.S. Lee, Sens Actuat. B: Chem. 264, 45 (2018)
N.A. Vazquez-Mera, J.R. Otaegui, R.S. Sanchez, G. Prats, G. Guirado, D. Ruiz-Molina, C. Roscini, J. Hernando, A.C.S. Appl, Mater. Interfaces 11, 17751 (2019)
R.P. Ollier, V.A. Alvarez, Colloid Surf. A-Physicochem. Eng. Asp. 520, 872 (2017)
G.T. Pak, H. Kim, T.S. Lee, Bull. Korean Chem. Soc. 42, 124 (2021)
Acknowledgements
This work was supported by the National Research Foundation (NRF) grant funded by the Korean government (MSIT) (2020R1A5A8017671 and 2023R1A2C2006046).
Author information
Authors and Affiliations
Contributions
GTP; methodology, formal analysis, writing—original draft preparation, SJ; validation, writing—review and editing, TSL; funding acquisition, project administration, conceptualization. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13233_2024_248_MOESM1_ESM.docx
Supplementary file1 (DOCX 630 KB) Experimental procedure for the preparation of synthetic methods for monomers and polymers, photographs, IR spectra, and size distribution of MFMs. The materials are available via the Internet at http://www.springer.com/13233. Supporting Information should be attached at the end of the article or separated file.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pak, G.T., Jo, S. & Lee, T.S. Achieving white light emission using melamine–formaldehyde microcapsules containing three fluorescence color-emitting conjugated polymers. Macromol. Res. 32, 597–603 (2024). https://doi.org/10.1007/s13233-024-00248-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-024-00248-8