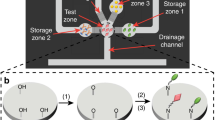
Abstract
Recently, with the development of microfluidic chips, attempts to integrate cell culture and biomedical material testing functions into a single chip have increased to supplement experimental animal models. Among the evaluations of biomaterials, immunogenicity testing, a current priority, is attracting attention. In this study, we developed a simple and easy-to-handle immunogenicity-testing cell chip to evaluate the immunogenicity of biomaterials. On this chip, macrophages were introduced as immunogenicity indicators, and a micro-paper-based analytical device (µPAD) was used for optical analysis. Macrophages are present in all parts of the body and mediate immune reactions against body implants or bio-derived substances, resulting in the production of hydrogen peroxide. In the cell chamber of the developed cell chip, macrophages grow and react to immunogenic materials. Activated macrophages secrete hydrogen peroxide, which is then transferred to the PAD with single-finger actuation. The hydrogen peroxide molecules reaching the PAD detection zone react with the colorimetric detection substrate, resulting in a color that corresponds to the hydrogen peroxide concentration. With the developed testing chip, the immunogenicity of biomaterials can be determined before administration.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Primiceri, E., Chiriacò, M.S., Rinaldi, R., Maruccio, G.: Cell chips as new tools for cell biology–results, perspectives and opportunities. Lab Chip 13, 3789–3802 (2013)
Dou, J., Mao, S., Li, H., Lin, J.-M.: Combination stiffness gradient with chemical stimulation directs glioma cell migration on a microfluidic chip. Anal. Chem. 92, 892–898 (2020)
Ceccacci, A.C., et al.: Blu-Ray-based micromechanical characterization platform for biopolymer degradation assessment. Sens. Actuators B Chem. 241, 1303–1309 (2017)
Li, Z., Seker, E.: Configurable microfluidic platform for investigating therapeutic delivery from biomedical device coatings. Lab Chip 17, 3331–3337 (2017)
Esch, E.W., Bahinski, A., Huh, D.: Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14, 248–260 (2015)
Zhai, J., et al.: Cancer drug screening with an on-chip multi-drug dispenser in digital microfluidics. Lab Chip 21, 4749–4759 (2021)
Jabbar, F., Kim, Y.-S., Lee, S.H.: Biological influence of pulmonary disease conditions induced by particulate matter on microfluidic lung chips. BioChip J. 16, 305–316 (2022)
Kang, S.-M.: Recent advances in microfluidic-based microphysiological systems. BioChip J. 16, 13–26 (2022)
Wiles, K., Fishman, J.M., De Coppi, P., Birchall, M.A.: The host immune response to tissue-engineered organs: crrent problems and future directions. Tissue Eng. Part B Rev. 22, 208–219 (2016)
Petrus-Reurer, S., et al.: Immunological considerations and challenges for regenerative cellular therapies. Commun. Biol. 4, 798 (2021)
Sadtler, K., et al.: Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 1, 16040 (2016)
Moore, E.M., Maestas, D.R., Jr., Comeau, H.Y., Elisseeff, J.H.: The immune system and its contribution to variability in regenerative medicine. Tissue Eng. Part B: Rev. 27, 39–47 (2021)
Hirayama, D., Iida, T., Nakase, H.: The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 19, 92 (2018)
Zhang, C., Yang, M., Ericsson, A.C.: Function of macrophages in disease: current understanding on molecular mechanisms. Front. Immunol. 12, 620510 (2021)
Stuart, L.M., Ezekowitz, R.A.B.: Phagocytosis: elegant complexity. Immunity 22, 539–550 (2005)
Elhelu, M.A.: The role of macrophages in immunology. J. Natl. Med. Assoc. 75, 314–317 (1983)
Uribe-Querol, E., Rosales, C.: Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 11, 1066 (2020)
Mosser, D.M., Hamidzadeh, K., Goncalves, R.: Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 18, 579–587 (2021)
Park, S.-J., et al.: Imaging inflammation using an activated macrophage probe with Slc18b1 as the activation-selective gating target. Nat. Commun. 10, 1111 (2019)
Vázquez-Romero, A., et al.: Multicomponent reactions for de novo synthesis of BODIPY probes: in vivo imaging of phagocytic macrophages. J. Am. Chem. Soc. 135, 16018–16021 (2013)
Saxena, R.K., Vallyathan, V., Lewis, D.M.: Evidence for lipopolysaccharide-induced differentiation of RAW264.7 murine macrophage cell line into dendritic like cells. J. Biosci. 28(129), 134 (2003)
Uchikura, K., et al.: Lipopolysaccharides induced increases in Fas ligand expression by Kupffer cells via mechanisms dependent on reactive oxygen species. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G620–G626 (2004)
Droge, W.: Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002)
Valko, M., et al.: Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 (2007)
Herb, M., Schramm, M.: Functions of ROS in macrophages and antimicrobial immunity. Antioxidants 10, 313 (2021)
Milkovic, L., Gasparovic, A.C., Cindric, M., Mouthuy, P.-A., Zarkovic, N.: Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 8, 793 (2019)
Forman, H.J., Ursini, F., Maiorino, M.: An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 73, 2–9 (2014)
Lennicke, C., Rahn, J., Lichtenfels, R., Wessjohann, L.A., Seliger, B.: Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 13, 39 (2015)
Zhang, J., et al.: ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 4350965 (2016)
Redza-Dutordoir, M., Averill-Bates, D.A.: Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 1863, 2977–2992 (2019)
Torino, S., Corrado, B., Iodice, M., Coppola, G.: PDMS-based microfluidic devices for cell culture. Inventions 3, 65 (2018)
Vickerman, V., Blundo, J., Chung, S., Kamm, R.: esign, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8, 1468–1477 (2008)
Lee, S.Y., et al.: Development of gut-mucus chip for intestinal absorption study. BioChip J. (2023). https://doi.org/10.1007/s13206-023-00097-0
Park, Y.M., et al.: Integrated pumpless microfluidic chip for the detection of foodborne pathogens by polymerase chain reaction and electrochemical analysis. Sens. Actuators B Chem. 329, 129130 (2021)
Liebisch, F., Weltin, A., Marzioch, J., Urban, G.A., Kieninger, J.: Zero-consumption Clark-type microsensor for oxygen monitoring in cell culture and organ-on-chip systems. Sens. Actuators B Chem. 322, 128652 (2020)
Chun, H.J., Park, Y.M., Han, Y.D., Jang, Y.H., Yoon, H.C.: Paper-based glucose biosensing system utilizing a smartphone as a signal reader. BioChip J. 8, 218–226 (2014)
Liu, M.-M., et al.: MoOx quantum dots with peroxidase-like activity on microfluidic paper-based analytical device for rapid colorimetric detection of H2O2 released from PC12 cells. Sens. Actuators B Chem. 305, 127512 (2020)
Lim, H., Jafry, A.T., Lee, J.: Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 24, 2869 (2019)
Im, S.H., et al.: An animal cell culture monitoring system using asmartphone-mountable paper-based analytical device. Sens. Actuators B Chem. 229, 166–173 (2016)
Kim, S., Lee, J.-H.: Current advances in paper-based biosensor technologies for rapid COVID-19 diagnosis. BioChip 16, 376–396 (2022)
Acknowledgements
This work was supported by the Creative Materials Discovery Program (NRF-2019M3D1A1078943) and research grants (NRF-2019R1A6A1A11051471, NRF-2021R1A2C3004180) funded by the National Research Foundation of Korea. H.C.Y also acknowledges the support from the Commercialization Promotion Agency for R & D Outcomes grant funded by the Korean government (2021N100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, K.W., Yang, E.K., Oh, Y. et al. Immunogenicity Monitoring Cell Chip Incorporating Finger-Actuated Microfluidic and Colorimetric Paper-Based Analytical Functions. BioChip J 17, 329–339 (2023). https://doi.org/10.1007/s13206-023-00111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-023-00111-5