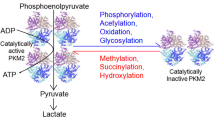
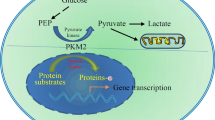
Abstract
Pyruvate kinase M2 (PKM2), a subtype of pyruvate kinase (PK), has been shown to play an important role in the development of cancer. It regulates the last step of glycolytic pathway. PKM2 has both pyruvate kinase and protein kinase activity, and the conversion of these two functions of PKM2 depends on the mutual change of dimer and tetramer. The dimerization of PKM2 can promote the proliferation and growth of tumor cells, so inhibiting the dimerization of PKM2 is essential to curing cancer. The aggregation of PKM2 is regulated by both endogenous and exogenous cofactors as well as post-translational modification (PTM). Although there are many studies on the different aggregation of PKM2 in the process of tumor development, there are few summaries in recent years. In this review, we first introduce the role of PKM2 in various biological processes of tumor growth. Then, we summarize the aggregation regulation mechanism of PKM2 by various endogenous cofactors such as Fructose-1, 6-diphosphate (FBP), various amino acids, and post-translational modification (PTMs). Finally, the related inhibitors and agonists of PKM2 are summarized to provide reference for regulating PKM2 aggregation in the treatment of cancer in the future.










Similar content being viewed by others
Data availability
Not applicable.
References
Abeywardana T, Oh M, Jiang L et al (2018) CARM1 suppresses de novo serine synthesis by promoting PKM2 activity. J Biol Chem 293:15290–15303. https://doi.org/10.1074/jbc.RA118.004512
Alquraishi M, Puckett DL, Alani DS et al (2019) Pyruvate kinase M2: A simple molecule with complex functions. Free Radical Biol Med 143:176–192. https://doi.org/10.1016/j.freeradbiomed.2019.08.007
Anastasiou D, Poulogiannis G, Asara JM et al (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science (New York, N.Y.) 334 1278–1283 https://doi.org/10.1126/science.1211485
An S, Huang L, Miao P et al (2018) Small ubiquitin-like modifier 1 modification of pyruvate kinase M2 promotes aerobic glycolysis and cell proliferation in A549 human lung cancer cells. Onco Targets Ther 11:2097–2109. https://doi.org/10.2147/ott.S156918
Anastasiou D, Yu Y, Israelsen WJ et al (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8:839–847. https://doi.org/10.1038/nchembio.1060
Azoitei N, Becher A, Steinestel K et al (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer 15:3. https://doi.org/10.1186/s12943-015-0490-2
Boussiotis VA (2016) Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 375:1767–1778. https://doi.org/10.1056/NEJMra1514296
Chaneton B, Hillmann P, Zheng L et al (2012) Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491:458–462. https://doi.org/10.1038/nature11540
Chen J, Jiang Z, Wang B et al (2012) Vitamin K(3) and K(5) are inhibitors of tumor pyruvate kinase M2. Cancer Lett 316:204–210. https://doi.org/10.1016/j.canlet.2011.10.039
Chen M, Liu H, Li Z et al (2021) Mechanism of PKM2 affecting cancer immunity and metabolism in Tumor Microenvironment, Journal of. Cancer 12:3566–3574. https://doi.org/10.7150/jca.54430
Dai Y, Liu P, Wen W et al (2023) Sarsasapogenin, a principal active component absorbed into blood of total saponins of Anemarrhena, attenuates proliferation and invasion in rheumatoid arthritis fibroblast-like synoviocytes through downregulating PKM2 inhibited pathological glycolysis. Phytotherapy research : PTR. https://doi.org/10.1002/ptr.7712
David CJ, Chen M, Assanah M et al (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463:364–368. https://doi.org/10.1038/nature08697
Ding Y, Xue Q, Liu S et al (2020) Identification of Parthenolide Dimers as Activators of Pyruvate Kinase M2 in Xenografts of Glioblastoma Multiforme in Vivo. J Med Chem 63:1597–1611. https://doi.org/10.1021/acs.jmedchem.9b01328
Dombrauckas JD, Santarsiero BD, Mesecar AD (2005) Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44:9417–9429. https://doi.org/10.1021/bi0474923
Du Y, Dong S, Jiang W, et al. (2023) Integration of Single-Cell RNA Sequencing and Bulk RNA Sequencing Reveals That TAM2-Driven Genes Affect Immunotherapeutic Response and Prognosis in Pancreatic Cancer, Int J Mol Sci 24 https://doi.org/10.3390/ijms241612787
Feng J, Dai W, Mao Y et al (2020) Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res: CR 39:24. https://doi.org/10.1186/s13046-020-1528-x
Giannoni E, Taddei ML, Morandi A, et al. (2015) Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs oxphos and prostate cancer metastatic spread, Oncotarget 6 24061–24074 https://doi.org/10.18632/oncotarget.4448
Gordon SR, Maute RL, Dulken BW et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545:495–499. https://doi.org/10.1038/nature22396
Gui DY, Lewis CA, Vander Heiden MG (2013) Allosteric regulation of PKM2 allows cellular adaptation to different physiological states, Sci Signal 6 pe7 https://doi.org/10.1126/scisignal.2003925
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
He D, Feng H, Sundberg B et al (2022) Methionine oxidation activates pyruvate kinase M2 to promote pancreatic cancer metastasis. Mol Cell 82:3045-3060.e3011. https://doi.org/10.1016/j.molcel.2022.06.005
Hitosugi T, Kang S, Vander Heiden MG et al (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2:ra73. https://doi.org/10.1126/scisignal.2000431
Hou PP, Luo LJ, Chen HZ et al (2020) Ectosomal PKM2 Promotes HCC by Inducing Macrophage Differentiation and Remodeling the Tumor Microenvironment. Mol Cell 78:1192-1206.e1110. https://doi.org/10.1016/j.molcel.2020.05.004
Jiang JK, Walsh MJ, Brimacombe KR, et al. (2010) ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. Probe Reports from the NIH Molecular Libraries Program, National Center for Biotechnology Information (US), Bethesda (MD). https://www.ncbi.nlm.nih.gov/books/NBK153222/
Jurisic V (2020) Multiomic analysis of cytokines in immuno-oncology. Exp Rev Proteomics 17:663–674. https://doi.org/10.1080/14789450.2020.1845654
Jurisić V, Konjević G, Jancić-Nedeljkov R et al (2004) The comparison of spontaneous LDH release activity from cultured PBMC with sera LDH activity in non-Hodgkin’s lymphoma patients. Med Oncol (Northwood, London, England) 21:179–185. https://doi.org/10.1385/mo:21:2:179
Jurisic V, Radenkovic S, Konjevic G (2015) The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv Exp Med Biol 867:115–124. https://doi.org/10.1007/978-94-017-7215-0_8
Keller KE, Tan IS, Lee YS (2012) SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Sci (New York, N.Y.) 338:1069–1072. https://doi.org/10.1126/science.1224409
Keller KE, Doctor ZM, Dwyer ZW et al (2014) SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell 53:700–709. https://doi.org/10.1016/j.molcel.2014.02.015
Kim DJ, Park YS, Kang MG et al (2015) Pyruvate kinase isoenzyme M2 is a therapeutic target of gemcitabine-resistant pancreatic cancer cells. Exp Cell Res 336:119–129. https://doi.org/10.1016/j.yexcr.2015.05.017
Konjević G, Jurisić V, Spuzić I (2001) Association of NK cell dysfunction with changes in LDH characteristics of peripheral blood lymphocytes (PBL) in breast cancer patients. Breast Cancer Res Treat 66:255–263. https://doi.org/10.1023/a:1010602822483
Konjević GM, Vuletić AM, Mirjačić Martinović KM et al (2019) The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 117:30–40. https://doi.org/10.1016/j.cyto.2019.02.001
Li C, Zhao Z, Zhou Z et al (2016) PKM2 Promotes Cell Survival and Invasion Under Metabolic Stress by Enhancing Warburg Effect in Pancreatic Ductal Adenocarcinoma. Dig Dis Sci 61:767–773. https://doi.org/10.1007/s10620-015-3931-2
Li X, Deng S, Liu M et al (2018) The responsively decreased PKM2 facilitates the survival of pancreatic cancer cells in hypoglucose. Cell Death Dis 9:133. https://doi.org/10.1038/s41419-017-0158-5
Li TE, Wang S, Shen XT et al (2020) PKM2 Drives Hepatocellular Carcinoma Progression by Inducing Immunosuppressive Microenvironment. Front Immunol 11:589997. https://doi.org/10.3389/fimmu.2020.589997
Li L, Song D, Qi L et al (2021) Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett 520:143–159. https://doi.org/10.1016/j.canlet.2021.07.014
Liang J, Cao R, Wang X et al (2017) Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res 27:329–351. https://doi.org/10.1038/cr.2016.159
Liu J, Wu N, Ma L et al (2014) Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms. PLoS ONE 9:e91606. https://doi.org/10.1371/journal.pone.0091606
Liu F, Ma F, Wang Y et al (2017) PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat Cell Biol 19:1358–1370. https://doi.org/10.1038/ncb3630
Luo W, Semenza GL (2012) Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab 23:560–566. https://doi.org/10.1016/j.tem.2012.06.010
Lv L, Li D, Zhao D et al (2011) Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell 42:719–730. https://doi.org/10.1016/j.molcel.2011.04.025
Lv L, Xu YP, Zhao D et al (2013) Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell 52:340–352. https://doi.org/10.1016/j.molcel.2013.09.004
Macpherson JA, Theisen A, Masino L, et al. (2019) Functional cross-talk between allosteric effects of activating and inhibiting ligands underlies PKM2 regulation, eLife 8 https://doi.org/10.7554/eLife.45068
Mirjačić Martinović K, Vuletić A, Tišma Miletić N et al (2023) Circulating cytokine dynamics as potential biomarker of response to anti-PD-1 immunotherapy in BRAFwt MM patients. Transl Oncol 38:101799. https://doi.org/10.1016/j.tranon.2023.101799
Morgan HP, O’Reilly FJ, Wear MA et al (2013) M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci USA 110:5881–5886. https://doi.org/10.1073/pnas.1217157110
Nandi S, Dey M (2020) Biochemical and structural insights into how amino acids regulate pyruvate kinase muscle isoform 2. J Biol Chem 295:5390–5403. https://doi.org/10.1074/jbc.RA120.013030
Nandi S, Razzaghi M, Srivastava D et al (2020) Structural basis for allosteric regulation of pyruvate kinase M2 by phosphorylation and acetylation. J Biol Chem 295:17425–17440. https://doi.org/10.1074/jbc.RA120.015800
Noguchi T, Inoue H, Tanaka T (1986) The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 261:13807–13812
Panieri E, Santoro MM (2016) ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7:e2253. https://doi.org/10.1038/cddis.2016.105
Park SH, Ozden O, Liu G et al (2016) SIRT2-Mediated Deacetylation and Tetramerization of Pyruvate Kinase Directs Glycolysis and Tumor Growth. Can Res 76:3802–3812. https://doi.org/10.1158/0008-5472.Can-15-2498
Popović B, Jekić B, Novaković I et al (2007) Bcl-2 expression in oral squamous cell carcinoma. Ann N Y Acad Sci 1095:19–25. https://doi.org/10.1196/annals.1397.003
Qi H, Ning X, Yu C et al (2019) Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis 10:170. https://doi.org/10.1038/s41419-018-1271-9
Rajala RVS (2020) Aerobic Glycolysis in the Retina: Functional Roles of Pyruvate Kinase Isoforms. Front Cell Dev Biol 8:266. https://doi.org/10.3389/fcell.2020.00266
Ren R, Guo J, Shi J et al (2020) PKM2 regulates angiogenesis of VR-EPCs through modulating glycolysis, mitochondrial fission, and fusion. J Cell Physiol 235:6204–6217. https://doi.org/10.1002/jcp.29549
Saleme B, Gurtu V, Zhang Y, et al. (2019) Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity, Science translational medicine 11 https://doi.org/10.1126/scitranslmed.aau8866
Singh JP, Qian K, Lee JS et al (2020) O-GlcNAcase targets pyruvate kinase M2 to regulate tumor growth. Oncogene 39:560–573. https://doi.org/10.1038/s41388-019-0975-3
Sizemore ST, Zhang M, Cho JH et al (2018) Pyruvate kinase M2 regulates homologous recombination-mediated DNA double-strand break repair. Cell Res 28:1090–1102. https://doi.org/10.1038/s41422-018-0086-7
Srivastava D, Nandi S, Dey M (2019) Mechanistic and Structural Insights into Cysteine-Mediated Inhibition of Pyruvate Kinase Muscle Isoform 2. Biochemistry 58:3669–3682. https://doi.org/10.1021/acs.biochem.9b00349
Tang JC, Zhao J, Long F et al (2018) Efficacy of Shikonin against Esophageal Cancer Cells and its possible mechanisms in vitro and in vivo. J Cancer 9:32–40. https://doi.org/10.7150/jca.21224
Tao T, Su Q, Xu S et al (2019) Down-regulation of PKM2 decreases FASN expression in bladder cancer cells through AKT/mTOR/SREBP-1c axis. J Cell Physiol 234:3088–3104. https://doi.org/10.1002/jcp.27129
Villar VH, Merhi F, Djavaheri-Mergny M et al (2015) Glutaminolysis and autophagy in cancer. Autophagy 11:1198–1208. https://doi.org/10.1080/15548627.2015.1053680
Wang P, Sun C, Zhu T et al (2015) Structural insight into mechanisms for dynamic regulation of PKM2. Protein Cell 6:275–287. https://doi.org/10.1007/s13238-015-0132-x
Wang F, Wang K, Xu W et al (2017) SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep 19:2331–2344. https://doi.org/10.1016/j.celrep.2017.05.065
Wang Y, Liu J, ** X et al (2017) O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci USA 114:13732–13737. https://doi.org/10.1073/pnas.1704145115
Wang Y, Hao F, Nan Y et al (2018) PKM2 Inhibitor Shikonin Overcomes the Cisplatin Resistance in Bladder Cancer by Inducing Necroptosis. Int J Biol Sci 14:1883–1891. https://doi.org/10.7150/ijbs.27854
Wang D, Zhao C, Xu F et al (2021) Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics 11:2860–2875. https://doi.org/10.7150/thno.51797
Wang Y, Shu H, Liu J et al (2022) EGF promotes PKM2 O-GlcNAcylation by stimulating O-GlcNAc transferase phosphorylation at Y976 and their subsequent association. J Biol Chem 298:102340. https://doi.org/10.1016/j.jbc.2022.102340
Wang J, Yang P, Yu T et al (2022) Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci 18:6210–6225. https://doi.org/10.7150/ijbs.75434
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530. https://doi.org/10.1085/jgp.8.6.519
Wong N, De Melo J, Tang D (2013) PKM2, a Central Point of Regulation in Cancer Metabolism. Int J Cell Biol 2013:242513. https://doi.org/10.1155/2013/242513
**a L, Jiang Y, Zhang XH et al (2021) SUMOylation disassembles the tetrameric pyruvate kinase M2 to block myeloid differentiation of leukemia cells. Cell Death Dis 12:101. https://doi.org/10.1038/s41419-021-03400-9
**a Y, Wang X, Liu Y et al (2022) PKM2 Is Essential for Bladder Cancer Growth and Maintenance. Can Res 82:571–585. https://doi.org/10.1158/0008-5472.Can-21-0403
**a Q, Jia J, Hu C et al (2022) Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene 41:865–877. https://doi.org/10.1038/s41388-021-02133-5
** G et al (2017) Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth. Oncotarget 8:6984–6993. https://doi.org/10.18632/oncotarget.14346
Xu Q, Liu LZ, Yin Y et al (2015) Regulatory circuit of PKM2/NF-κB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene 34:5482–5493. https://doi.org/10.1038/onc.2015.6
Xu Q, Tu J, Dou C et al (2017) HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer 16:178. https://doi.org/10.1186/s12943-017-0748-y
Yan M, Chakravarthy S, Tokuda JM et al (2016) Succinyl-5-aminoimidazole-4-carboxamide-1-ribose 5’-Phosphate (SAICAR) Activates Pyruvate Kinase Isoform M2 (PKM2) in Its Dimeric Form. Biochemistry 55:4731–4736. https://doi.org/10.1021/acs.biochem.6b00658
Yan XL, Zhang XB, Ao R et al (2017) Effects of shRNA-Mediated Silencing of PKM2 Gene on Aerobic Glycolysis, Cell Migration, Cell Invasion, and Apoptosis in Colorectal Cancer Cells. J Cell Biochem 118:4792–4803. https://doi.org/10.1002/jcb.26148
Yang W, Zheng Y, **a Y et al (2012) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14:1295–1304. https://doi.org/10.1038/ncb2629
Yu Z, Zhao X, Huang L et al (2013) Proviral insertion in murine lymphomas 2 (PIM2) oncogene phosphorylates pyruvate kinase M2 (PKM2) and promotes glycolysis in cancer cells. J Biol Chem 288:35406–35416. https://doi.org/10.1074/jbc.M113.508226
Yu Z, Huang L, Qiao P et al (2016) PKM2 Thr454 phosphorylation increases its nuclear translocation and promotes xenograft tumor growth in A549 human lung cancer cells. Biochem Biophys Res Commun 473:953–958. https://doi.org/10.1016/j.bbrc.2016.03.160
Yuan M, McNae IW, Chen Y et al (2018) An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor. Biochem J 475:1821–1837. https://doi.org/10.1042/bcj20180171
Zhang W, Guo X, Ren J et al (1987) GCN5-mediated PKM2 acetylation participates in benzene-induced hematotoxicity through regulating glycolysis and inflammation via p-Stat3/IL17A axis, Environmental pollution (Barking. Essex 295(2022):118708. https://doi.org/10.1016/j.envpol.2021.118708
Zhang D, Tang Z, Huang H et al (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574:575–580. https://doi.org/10.1038/s41586-019-1678-1
Zhang W, Zhang X, Huang S et al (2021) FOXM1D potentiates PKM2-mediated tumor glycolysis and angiogenesis. Mol Oncol 15:1466–1485. https://doi.org/10.1002/1878-0261.12879
Zheng S, Liu Q, Liu T et al (2021) Posttranslational modification of pyruvate kinase type M2 (PKM2): novel regulation of its biological roles to be further discovered. J Physiol Biochem 77:355–363. https://doi.org/10.1007/s13105-021-00813-0
Zhou Z, Li M, Zhang L et al (2018) Oncogenic Kinase-Induced PKM2 Tyrosine 105 Phosphorylation Converts Nononcogenic PKM2 to a Tumor Promoter and Induces Cancer Stem-like Cells. Can Res 78:2248–2261. https://doi.org/10.1158/0008-5472.Can-17-2726
Zhou Y, Huang Z, Su J et al (2020) Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int J Cancer 147:139–151. https://doi.org/10.1002/ijc.32756
Zhou S, Li D, **ao D et al (2022) Inhibition of PKM2 Enhances Sensitivity of Olaparib to Ovarian Cancer Cells and Induces DNA Damage. Int J Biol Sci 18:1555–1568. https://doi.org/10.7150/ijbs.62947
Acknowledgements
We would like to thank Dr. Joseph Elliot at the University of Kansas for her assistance with English language and grammatical editing of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81930114 and 82322074), Key-Area Research and Development Program of Guangdong Province (Grant No. 2020B1111100004), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Laboratory, Grant No. 2020B1212030006).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. Bingxin Wu, Investigation, Writing—Original Draft. **aojun Teng, Investigation. Huan Lan, Visualization. Zuhui Liang, Visualization, Investigation. Caiyan Wang, Writing—Review & Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
Compared with normal tissues, PKM2 is highly expressed in cancer cells and affects the occurrence and development of cancer cells.
The conformational changes of PKM2 affect the survival and development of cancer cells.
Targeted regulation of PKM2 conformational changes is an effective means for the treatment of cancer in the future.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, B., Liang, Z., Lan, H. et al. The role of PKM2 in cancer progression and its structural and biological basis. J Physiol Biochem 80, 261–275 (2024). https://doi.org/10.1007/s13105-024-01007-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-024-01007-0