Abstract
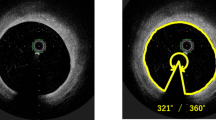
Intravascular optical coherence tomography is a high-resolution intracoronary imaging modality, providing a microscopic image of intravascular features. However, it has lower penetration depth than intravascular ultrasound. Recently, a second-generation optical frequency-domain imaging (OFDI) technique has been developed to provide greater penetration depth and faster pullback speed. However, there is little evidence supporting the efficacy of OFDI in patients with peripheral artery disease (PAD) undergoing endovascular treatment (EVT). We aimed to evaluate the ability of OFDI to visualize vessel walls from the superficial femoral artery (SFA) to the below-knee (BK) arteries, as well as the coronary arteries. This clinical trial is a single-center, single-arm, open-label study to be conducted in Japan. A total of 20 patients will be enrolled in this study. The primary endpoint is to obtain a clear image of the intravascular features of the SFA and BK arteries, specifically the visualization of ≥ 270° of the vessel lumen in ≥ 16 out of 21 cross sections. Obtaining clear images in ≥ 85% of cases will be regarded as confirmation of the ability of OFDI to visualize vessel walls from the SFA to the BK arteries. This is the first clinical trial to be conducted accordance with good clinical practice to expand the indications of OFDI for PAD patients undergoing EVT in Japan. The result of this study will help provide another imaging option during EVT in daily practice.



Similar content being viewed by others
Change history
29 June 2020
In the original publication of the article, the author group was published without full names and one of the co-authors’ name was published incorrectly. The full names of author group and correct co-authors’ name are given in this Correction.
References
Rigatelli G, Cardaioli P, Giordan M. Endovascular treatment of femoro-popliteal obstructive disease. Minerva Cardioangiol. 2007;55:125–32.
Zdanowski Z, Albrechtsson U, Lundin A, Jonung T, Ribbe E, Thörne J, et al. Percutaneous transluminal angioplasty with or without stenting for femoropopliteal occlusions? A randomized controlled study. Int Angiol. 1999;18:251–5.
Vroegindeweij D, Vos LD, Tielbeek AV, Buth J, Bosch HC. Balloon angioplasty combined with primary stenting versus balloon angioplasty alone in femoropopliteal obstructions: a comparative randomized study. Cardiovasc Intervent Radiol. 1997;20:420–5.
Becquemin JP, Favre JP, Marzelle J, Nemoz C, Corsin C, Leizorovicz A. Systematic versus selective stent placement after superficial femoral artery balloon angioplasty: a multicenter prospective randomized study. J Vasc Surg. 2003;37:487–94.
Kawasaki D, Tsu**o T, Fujii K, Masutani M, Ohyanagi M, Masuyama T. Novel use of ultrasound guidance for recanalization of iliac, femoral, and popliteal arteries. Catheter Cardiovasc Interv. 2008;71:727–33.
Fujihara M, Kozuki A, Tsubakimoto Y, Takahara M, Shintani Y, Fukunaga M, et al. Lumen gain after endovascular therapy in calcified superficial femoral artery occlusive disease assessed by intravascular ultrasound (CODE Study). J Endovasc Ther. 2019;26:322–30.
Okamura T, Gonzalo N, Gutierrez-Chico JL, et al. Reproducibility of coronary Fourier domain optical coherence tomography: quantitative analysis of in vivo stented coronary arteries using three different software packages. EuroIntervention. 2010;6:371–9.
Miki K, Fujii K, Fukunaga M, Nishimura M, Horimatsu T, Saita T, et al. Strut coverage after paclitaxel-eluting stent implantation in the superficial femoral artery. JACC Cardiovasc Imaging. 2016;9:753–5.
Ali ZA, Maehara A, Genereux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–28.
Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139–47.
Otake H, Kubo T, Takahashi H, Shinke T, Okamura T, Hibi K, et al. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION Trial): results from the OPINION Imaging Study. JACC Cardiovasc Imaging. 2018;11:111–23.
Kozuki A, Shinke T, Otake H, Kijima Y, Masano T, Nagoshi R, et al. Optical coherence tomography study of chronic-phase vessel healing after implantation of bare metal and paclitaxel-eluting self-expanding nitinol stents in the superficial femoral artery. J Cardiol. 2016;67:424–9.
Miki K, Fujii K, Shibuya M, Fukunaga M, Imanaka T, Tamaru H, et al. Comparing the vascular response in implantation of self-expanding, bare metal nitinol stents or paclitaxel-eluting nitinol stents in superficial femoral artery lesions: a serial optical frequency domain imaging study. EuroIntervention. 2016;12:1551–8.
Miki K, Fujii K, Shibuya M, Fukunaga M, Imanaka T, Kawai K, et al. Impact of stent diameter on vascular response after self-expanding paclitaxel-eluting stent implantation in the superficial femoral artery. J Cardiol. 2017;70:346–52.
Eberhardt KM, Treitl M, Boesenecker K, Maxien D, Reiser M, Rieger J. Prospective evaluation of optical coherence tomography in lower limb arteries compared with intravascular ultrasound. J Vasc Interv Radiol. 2013;24:1499–508.
Marmagkiolis K, Lendel V, Cawich I, Leesar M, Feldman MD, Cilingiroglu M. Optical coherence tomography to guide below-the-knee endovascular interventions. Int J Cardiol. 2014;176:1336–8.
Jang IK, Tearney G, Bouma B. Visualization of tissue prolapse between coronary stent struts by optical coherence tomography: comparison with intravascular ultrasound. Circulation. 2001;104:2754.
Konishi A, Ho M, Shirai Y, Shirato H. First approval of improved medical device conditional on use-result survey in Japan—regulatory review of polymer-free drug-coated biofreedom coronary stent. Circul J. 2018;82:1487–90.
Pharmaceuticals and Medical Devices Agency. Optical coherence tomography review report. 2015. [in Japanese]. http://www.pmda.go.jp/medical_devices/2007/M200700004/250011000_21900BZX00779000_A100_1.pdf. Accessed 22 Sep 2019.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24.
Konishi A, Isobe S, Sato D. New regulatory framework for medical devices in Japan: current regulatory considerations regarding clinical studies. J Vasc Interv Radiol. 2018;29:657–60.
Acknowledgements
We are grateful to all of the staff involved in this clinical study.
Funding
This study project is funded by Terumo Corporation and supported by the Kobe Clinical and Translational Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Otake and Dr. Shinke serve as members of the advisory board of Abbott Vascular, Inc. The remaining authors have no conflicts of interest to declare.
Research involving human participants and/or animals
This protocol has been approved by the institutional review board of Kobe University Hospital (Protocol identification number: OFDI-01).
Informed consent
All patients must provide written informed consent. The principle investigator or sub-investigator will provide potential subjects with opportunities to ask questions and ample time to decide whether or not to provide consent, and should confirm sufficient understanding of the potential subjects about the contents of this study, before obtaining voluntary consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawamori, H., Konishi, A., Otake, H. et al. Efficacy of optical frequency-domain imaging in detecting peripheral artery disease: a single-center open-label, single-arm study protocol. Cardiovasc Interv and Ther 35, 385–391 (2020). https://doi.org/10.1007/s12928-019-00636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-019-00636-3