Abstract
Researchers are always coming up with new techniques and methods to ensure that drug delivery systems are secure, therapeutically efficient, and patient-compliant. Clay minerals are nanolayered silicates considered potential nanomaterials in medical research. They have been widely used as both active agents and excipients since ancient times for the treatment of various illnesses. Owing to their remarkable characteristics of high biocompatibility, loading capacity, retention capacity, rheological and swelling properties, and affordable price, they can be used as advanced carriers for the effective delivery of drugs by varying their release rate, boosting their stability, and improving their dissolution profile. Nowadays, drug delivery through the skin has received attention as an alternative to the oral route because the skin is a potential route to deliver local and systemic drugs as nanoparticles. In this research work, clonidine hydrochloride (CH) was chosen as a model drug that plays a major role in preventing post-operative pain (both acute and chronic). The epidural solution of clonidine is already available for cancer pain. The transdermal patches of clonidine are also available in different dosages, which are intended to be administered for 7 days to treat neuropathic pain. However, there is no availability of extended-release transdermal patches of clonidine to date for the treatment of several pains mentioned earlier. Therefore, an attempt was made to prepare such transdermal patches. The intercalation of CH into the interlayers of sodium montmorillonite (Na-MMT) nanoclay via three different methods was demonstrated. Freshly prepared CH/Na-MMT composites were utilized to fabricate the transdermal patches to examine the analgesic activity of the drug for an extended period in a single dose or not after entering systemic circulation via skin barriers. The composites and patches were characterized by Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD). Sustained release of CH from CH/Na-MMT composites was observed for nearly 1 month during the in vitro release experiment. The results obtained from the ex vivo skin permeation study, as well as in vivo analgesic activity using those patches, corroborated the outcomes of the in vitro release study. Different kinetic models were applied to the ex vivo dissolution data to determine the best-fit kinetic model accompanied by drug release in both burst and sustained release periods.
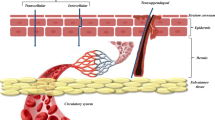
Graphical Abstract







Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Kumar, A., Maitra, S., Khanna, P., & Baidya, D. K. (2014). Clonidine for management of chronic pain: A brief review of the current evidences. Saudi Journal of Anaesthesia, 8(1), 92–96. https://doi.org/10.4103/1658-354X.125955
Hughes, P. L., & Morse, R. M. (1985). Use of clonidine in a mixed-drug detoxification regimen: Possibility of masking of clinical signs of sedative withdrawal. Mayo Clinic Proceedings, 60(1), 47–49. https://doi.org/10.1016/S0025-6196(12)65281-1
Wrzosek, A., Woron, J., Dobrogowski, J., Jakowicka-Wordliczek, J., & Wordliczek, J. (2015). Topical clonidine for neuropathic pain. Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd. https://doi.org/10.1002/14651858.CD010967.pub2
Wang, J. G., Belley-Coté, E., Burry, L., Duffett, M., Karachi, T., Perri, D., Alhazzani, W., D’Aragon, F., Wunsch, H., & Rochwerg, B. (2017). Clonidine for sedation in the critically ill: A systematic review and meta-analysis. Critical Care, 21(1), 1–11. https://doi.org/10.1186/s13054-017-1610-8
Singh, R., Chopra, N., Choubey, S., Tripathi, R., Prabhakar, & Mishra, A. (2014). Role of clonidine as adjuvant to intrathecal bupivacaine in patients undergoing lower abdominal surgery: A randomized control study. Anesthesia: Essays and Researches, 8(3), 307. https://doi.org/10.4103/0259-1162.143119
Glynn, C., Dawson, D., & Sanders, R. (1988). A double-blind comparison between epidural morphine and epidural clonidine in patients with chronic non-cancer pain. Pain Vol. 34.
Roh, D. H., Kim, H. W., Yoon, S. Y., Seo, H. S., Kwon, Y. B., Han, H. J., … Lee, J. H. (2008). Intrathecal clonidine suppresses phosphorylation of the n-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesthesia and Analgesia, 107(2), 693–700. https://doi.org/10.1213/ane.0b013e31817e7319
Vasseur, B., Dufour, A., Houdas, L., Goodwin, H., Harries, K., Emul, N. Y., & Hutchings, S. (2017). Comparison of the systemic and local pharmacokinetics of clonidine mucoadhesive buccal tablets with reference clonidine oral tablets in healthy volunteers: An open-label randomised cross-over trial. Advances in Therapy, 34(8), 2022–2032. https://doi.org/10.1007/s12325-017-0585-9
Jain, R., Segal, S., Kollins, S. H., & Khayrallah, M. (2011). Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 50(2), 171–179. https://doi.org/10.1016/j.jaac.2010.11.005
Weber, M. A., Drayer, J. I. M., McMahon, F. G., Hamburger, R., Shah, A. R., & Kirk, L. N. (1984). Transdermal administration of clonidine for treatment of high BP. Archives of Internal Medicine, 144(6), 1211–1213. https://doi.org/10.1001/archinte.1984.00350180139020
Roelants, F., Lavand’homme, P. M., & Mercier-Fuzier, V. (2005). Epidural administration of neostigmine and clonidine to induce labor analgesia evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology (Vol. 102). Retrieved from www.anesthesiology.org
Ackerman, L. L., Follett, K. A., & Rosenquist, R. W. (2003). Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. Journal of Pain and Symptom Management, 26(1), 668–677. https://doi.org/10.1016/S0885-3924(03)00144-1
Behrman, A., & Goertemoeller, S. (2007). A sticky situation: Toxicity of clonidine and fentanyl transdermal patches in pediatrics. Journal of Emergency Nursing, 33(3), 290–293. https://doi.org/10.1016/j.jen.2007.02.004
Bloch, R., Bousquet, P., Feldman, J., & Schwartz, J. (1973). The mechanism of action of clonidine. Frontiers in Catecholamine Research, 853–857. https://doi.org/10.1016/B978-0-08-017922-3.50162-3
Anderson, C., & Stone, T. W. (1974). On the mechanism of action of clonidine: Effects on single central neurones. British Journal of Pharmacology, 51(3), 359. https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.1974.tb10670.x
Neil, M. J. (2011). Clonidine: Clinical pharmacology and therapeutic use in pain management. Current Clinical Pharmacology, 6(4), 280–287. https://doi.org/10.2174/157488411798375886
Alam, M. I., Alam, N., Singh, V., Alam, M. S., Ali, M. S., Anwer, T., & Safhi, M. M. (2013). Type, preparation and evaluation of transdermal patch: A review. World Journal of Pharmacy and Pharmaceutical Sciences, 2(4), 2199–2233.
Purushotham, K., & Anie Vijetha, K. (2023). A review on transdermal drug delivery system. GSC Biological and Pharmaceutical Sciences, 22(2), 245–255. https://doi.org/10.30574/gscbps.2023.22.2.0053
Lohmann, V., Korner, F., Koch, J. O., Herian, U., Theilmann, L., & Bartenschlager, R. (1999). Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science, 285(5424), 110–113. https://doi.org/10.1126/science.285.5424.110
Gaignaux, A., Réeff, J., Siepmann, F., Siepmann, J., De Vriese, C., Goole, J., & Amighi, K. (2012). Development and evaluation of sustained-release clonidine-loaded PLGA microparticles. International Journal of Pharmaceutics, 437(1–2), 20–28. https://doi.org/10.1016/j.ijpharm.2012.08.006
Dubey, R., Dubey, B. K., Pandey, G. K., & Yadav, S. K. (2019). s):271-275 and characterization of alginate microbeads of clonidine hydrochloride by ionotropic gelation technique. Journal of Drug Delivery and Therapeutics, 9(2), 271–275. https://doi.org/10.22270/jddt.v9i2-s.2510
Dangre, P. V., Phad, R. D., Surana, S. J., & Chalikwar, S. S. (2019). Quality by design (QbD) assisted fabrication of fast dissolving buccal film for clonidine hydrochloride: Exploring the quality Attributes. Advances in Polymer Technology, 2019, 1–88. https://doi.org/10.1155/2019/3682402
Lanes, R., Recker, B., Fort, P., & Lifshitz, F. (1985). Low-dose oral clonidine: A simple and reliable growth hormone screening test for children. American Journal of Diseases of Children, 139(1), 87–88. https://doi.org/10.1001/archpedi.1985.02140030093040
Viseras, C., Carazo, E., Borrego-Sánchez, A., García-Villén, F., Sánchez-Espejo, R., Cerezo, P., & Aguzzi, C. (2019). Clay minerals in skin drug delivery. Clays and Clay Minerals, 67(1), 59–71. https://doi.org/10.1007/s42860-018-0003-7
Jayrajsinh, S., Shankar, G., Agrawal, Y. K., & Bakre, L. (2017). Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. Journal of Drug Delivery Science and Technology, 39, 200–209. https://doi.org/10.1016/j.jddst.2017.03.023
Dong, J., Cheng, Z., Tan, S., & Zhu, Q. (2021). Clay nanoparticles as pharmaceutical carriers in drug delivery systems. Taylor and Francis Ltd. https://doi.org/10.1080/17425247.2021.1862792
Damato, A., Vianello, F., Novelli, E., Balzan, S., Gianesella, M., Giaretta, E., & Gabai, G. (2022). Comprehensive review on the interactions of clay minerals with animal physiology and production. Frontiers in Veterinary Science, 9, 889612. https://doi.org/10.3389/fvets.2022.889612
Zhang, Y., Long, M., Huang, P., Yang, H., Chang, S., Hu, Y., ... & Mao, L. (2016). Emerging integrated nanoclay-facilitated drug delivery system for papillary thyroid cancer therapy. Scientific Reports, 6(1), 33335. https://doi.org/10.1038/srep33335
Park, J. H., Shin, H. J., Kim, M. H., Kim, J. S., Kang, N., Lee, J. Y., Kim, K. T., Lee, J. I., & Kim, D. D. (2016). Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. Journal of Pharmaceutical Investigation, 46, 363–375. https://doi.org/10.1007/s40005-016-0258-8
Salvé, J., Grégoire, B., Imbert, L., Hubert, F., Leitner, N. K. V., & Leloup, M. (2021). Design of hybrid chitosan-montmorillonite materials for water treatment: Study of the performance and stability. Chemical Engineering Journal Advances, 6, 100087. https://doi.org/10.1016/j.ceja.2021.100087
Madurai, S. L., Joseph, S. W., Mandal, A. B., Tsibouklis, J., & Reddy, B. S. R. (2011). Intestine-specific, oral delivery of captopril/montmorillonite: Formulation and release kinetics. Nanoscale Research Letters, 6(1), 1–8. https://doi.org/10.1007/s11671-010-9749-0
Adhikary, S., Al Hoque, A., Ray, M., Paul, S., Hossain, A., Goswami, S., & Dey, R. (2023). Investigation of paracetamol entrapped nanoporous silica nanoparticles in transdermal drug delivery system. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-023-04576-w
Banerjee, S., Chattopadhyay, P., Ghosh, A., Pathak, M. P., Singh, S., & Veer, V. (2013). Acute dermal irritation, sensitization, and acute toxicity studies of a transdermal patch for prophylaxis against ({+/-}) anatoxin-a poisoning. International journal of toxicology, 32(4), 308–313. https://doi.org/10.1177/1091581813489996
de Kumar, P., Mallick, S., Mukherjee, B., Sengupta, S., Pattnaik, S., & Chakraborty, S. (2011). Optimization of in-vitro permeation pattern of ketorolac tromethamine transdermal patches. Iranian Journal of Pharmaceutical Research, 10(2), 193–201. https://doi.org/10.22037/ijpr.2011.927
Ge, M., Li, Y., Zhu, C., Liang, G., Jahangir Alam, S. M., Hu, G., … Junaebur Rashid, M. (2021). Preparation of organic-modified magadiite–magnetic nanocomposite particles as an effective nanohybrid drug carrier material for cancer treatment and its properties of sustained release mechanism by Korsmeyer–Peppas kinetic model. Journal of Materials Science, 56(25), 14270–14286. https://doi.org/10.1007/s10853-021-06181-w
Daher Pereira, E., Thomas, S., Gomes de Souza Junior, F., da Silva Cardoso, J., Thode Filho, S., Corrêa da Costa, V., … Galal Aboelkheir, M. (2021). Study of controlled release of ibuprofen magnetic nanocomposites. Journal of Molecular Structure, 1232, 130067. https://doi.org/10.1016/J.MOLSTRUC.2021.130067
Fan, S.-H., Ali, N. A., & Basri, D. F. (2014). Evaluation of analgesic activity of the methanol extract from the galls of Quercus infectoria (Olivier) in rats. Evidence-Based Complementary and Alternative Medicine, 2014, 976764. https://doi.org/10.1155/2014/976764
Li, C., Fang, K., He, W., Li, K., Jiang, Y., & Li, J. (2021). Evaluation of chitosan-ferulic acid microcapsules for sustained drug delivery: Synthesis, characterizations, and release kinetics in vitro. Journal of Molecular Structure, 1227, 129353. https://doi.org/10.1016/J.MOLSTRUC.2020.129353
Uddin, F. (2018). Montmorillonite: An introduction to properties and utilization. In Current topics in the utilization of clay in industrial and medical applications. IntechOpen. https://doi.org/10.5772/intechopen.77987
Romano, E., Davies, L., & Brandán, S. A. (2017). Structural properties and FTIR-Raman spectra of the anti-hypertensive clonidine hydrochloride agent and their dimeric species. Journal of Molecular Structure, 1133, 226–235. https://doi.org/10.1016/j.molstruc.2016.12.008
Selvasudha, N., Dhanalekshmi, U.-M., Krishnaraj, S., Sundar, Y. H., Devi, N. S. D., & Sarathchandiran, I. (2020). Multifunctional clay in pharmaceuticals. In Clay science and technology. IntechOpen. https://doi.org/10.5772/intechopen.92408
Van Gheluwe, L., Chourpa, I., Gaigne, C., & Munnier, E. (2021). Polymer-based smart drug delivery systems for skin application and demonstration of stimuli-responsiveness. Polymers, 13(8), 1285. https://doi.org/10.3390/polym13081285
Aoi, W., Zou, X., **ao, J. B., & Marunaka, Y. (2020). Body fluid pH balance in metabolic health and possible benefits of dietary alkaline foods. Efood, 1(1), 12–23. https://doi.org/10.2991/efood.k.190924.001
Mohamed, W. S., Mostafa, A. B., & Nasr, H. E. (2014). Characterization and application of intercalated montmorillonite with verapamil and its polymethyl methacrylate nanocomposite in drug delivery. Polymer - Plastics Technology and Engineering, 53(14), 1425–1433. https://doi.org/10.1080/03602559.2014.909462
Akram, M. W., Jamshaid, H., Rehman, F. U., Zaeem, M., Khan, J. Z., & Zeb, A. (2022). Transfersomes: A revolutionary nanosystem for efficient transdermal drug delivery. AAPS PharmSciTech, 23, 1–18. https://doi.org/10.1208/s12249-021-02166-9
Lademann, J., Patzelt, A., Richter, H., Antoniou, C., Sterry, W., & Knorr, F. (2009). Determination of the cuticula thickness of human and porcine hairs and their potential influence on the penetration of nanoparticles into the hair follicles. Journal of Biomedical Optics, 14(2), 021014. https://doi.org/10.1117/1.3078813
Siepmann, J., & Peppas, N. A. (2011). Higuchi equation: Derivation, applications, use and misuse. International Journal of Pharmaceutics, 418(1), 6–12. https://doi.org/10.1016/j.ijpharm.2011.03.051
Elmas, A., Akyüz, G., Bergal, A., Andaç, M., & Andaç, Ö. (2020). Mathematical modelling of drug release. Research on Engineering Structures and Materials, 6(4), 327–350. https://doi.org/10.17515/resm2020.178na0122
Fu, Y., & Kao, W. J. (2010). Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opinion on Drug Delivery, 7(4), 429–444. https://doi.org/10.1517/17425241003602259
Rehage, G., Ernst, O., & Fuhrmann, J. (1970). Fickian and non-Fickian diffusion in high polymer systems. Discussions of the Faraday Society, 49, 208–221. https://doi.org/10.1039/DF9704900208
Bayer, I. S. (2023). Controlled drug release from nanoengineered polysaccharides. Pharmaceutics, 15(5), 1364. https://doi.org/10.3390/pharmaceutics15051364
Acknowledgements
We are thankful to the late Dr. Subrata Goswami, Department of Labour, ESI Institute of Pain Management, Kolkata, India, and Prof. (Dr.) Biswajit Mukherjee, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India, for their immense contribution to our research work.
Funding
Funding was provided by the Department of Higher Education, Science and Technology and Biotechnology, Government of West Bengal (File no: ST/P/S&T/6G-32/2017).
Author information
Authors and Affiliations
Contributions
Sourav Adhikary: conceptualization, data curation, formal analysis, investigation, methodology, and writing (original draft). Ashique Al Hoque: methodology and writing (review and editing). Manisheeta Ray: formal analysis and validation. Pritha Pal: investigation and validation. Mahua Ghosh Chaudhuri: resources and supervision. Rajib Dey: project administration, resources, and supervision.
Corresponding authors
Ethics declarations
Research Involving Humans and Animals Statement
Permission from the Institutional Animal Ethics Committee (IAEC) of Jadavpur University was received before commencing any animal experiments (JU-IAEC, Project proposal no.: JU-IAEC-22/11), under the norms of CPCSEA, Govt. of India (Jadavpur University Registration Number in CPCSEA: 1805/G.O./Re/S/15/CPCSEA). All experiments have been conducted following the guidelines of the Animal Ethics Committee of Jadavpur University.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adhikary, S., Al Hoque, A., Ray, M. et al. Tailored Transdermal Drug Delivery System for Pain Management: Development and Evaluation of Clonidine Hydrochloride/Sodium Montmorillonite Composite Patch. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01402-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01402-3