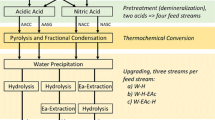
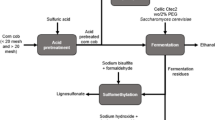
Abstract
Corncobs are lignocellulosic biomass that has received widespread attention as a promising raw material for bioethanol production because of its abundance and low-cost. They also relieve the predicament and ethics involved in using consumable food crops such as corn for bio-ethanol production when there are serious food shortages in many parts of the world. Corncobs have a high cellulose and hemicellulose content, high-molecular-weight constituents of lignocellulosic residues that can break down into simple sugars for microbial growth. Pretreatment of corncobs is necessary for lignin removal and the breakdown of cellulosic matter. Enzymes or acids act on the lignocelluloses to break down the cellulose into glucose monomers which are utilized by micro-organisms for conversion into ethanol through metabolic pathways. Large-scale production of bio-ethanol is stalled, attributed to the cost of production. The selection of a suitable pretreatment strategy will offset costs but is challenging as different methods offer different advantages over others in improving the overall efficiency of enzymatic saccharification and downstream processing costs. The current review accounts for strategies developed for bio-ethanol production using corncobs since pre-treating corncobs have been thoroughly researched and documented. The review hopes to establish the future of corncobs as a valuable commodity in the bioethanol industryby acting as knowledge or material source of the important processes to assist in designing a cost-effective ethanol production for industrial-scale production.
Graphic Abstract




Similar content being viewed by others
References
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production : a review. Bioresour. Technol. 83, 1–11 (2002). https://doi.org/10.1016/S0960-8524(01)00212-7
OECD/IEA, IRENA: Perspectives for the Energy Transition: Investment Needs for a Low-Carbon Energy System. Int. Energy Agency. p. 204 (2017)
Hamelinck, C.N., Van Hooijdonk, G., Faaij, A.P.C.: Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy. 28, 384–410 (2005). https://doi.org/10.1016/j.biombioe.2004.09.002
Anderson, J.E., Dicicco, D.M., Ginder, J.M., Kramer, U., Leone, T.G., Raney-Pablo, H.E., Wallington, T.J.: High octane number ethanol-gasoline blends: quantifying the potential benefits in the United States. Fuel 97, 585–594 (2012). https://doi.org/10.1016/j.fuel.2012.03.017
Demirbas, A.: Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 49, 2106–2116 (2008). https://doi.org/10.1016/j.enconman.2008.02.020
Demirbas, A.: Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 50, 14–34 (2009). https://doi.org/10.1016/j.enconman.2008.09.001
Bothast, R.J., Schlicher, M.A.: Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 67, 19–25 (2005)
Rosegrant, M.: Biofuels and Grain Prices: Impacts and Policy Responses, pp. 1–4. International Food Policy Research Institute, Washington, DC (2008)
Rosegrant, M.W., Zhu, T., Msangi, S., Sulser, T.: Global scenarios for biofuels: Impacts and implications. Rev. Agric. Econ. 30, 495–505 (2008). https://doi.org/10.1111/j.1467-9353.2008.00424.x
Mallia, E., Lewis, G.: Life cycle greenhouse gas emissions of electricity generation in the province of Ontario Canada. Int. J. Life Cycle Assess. 18, 377–391 (2013). https://doi.org/10.1007/s11367-012-0501-0
Bailey, R.W., Pickmere, S.E.: Alkali solubility of hemicelluloses in relation to delignification. Phytochemistry 14, 501–504 (1975). https://doi.org/10.1016/0031-9422(75)85117-X
Vanholme, R., Demedts, B., Morreel, K., Ralph, J., Boerjan, W.: Lignin biosynthesis and structure. Plant Physiol. 153, 895–905 (2010). https://doi.org/10.1104/pp.110.155119
Klemm, D., Heublein, B., Fink, H.P., Bohn, A.: Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 44, 3358–3393 (2005). https://doi.org/10.1002/anie.200460587
Scheller, H.V., Ulvskov, P.: Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289 (2010). https://doi.org/10.1146/annurev-arplant-042809-112315
Hayes, M.H.B., Mylotte, R., Swift, R.S.: Humin: Its Composition and Importance in Soil Organic Matter Advances in Agronomy, pp. 47–138. Academic Press, New York (2017)
Siqueira, G., Arantes, V., Saddler, J.N., Ferraz, A., Milagres, A.M.F.: Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol. Biofuels 10, 176 (2017). https://doi.org/10.1186/s13068-017-0860-7
Zhao, X., Zhang, L., Liu, D.: Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 6(4), 465–482 (2012)
Monsalve, G., John, F., Medina de Perez, V.I., Ruiz Colorado, A.A.: Ethanol production of banana shell and cassava starch. Dyna 150, 21–27 (2006)
Rowell, R.M.: Opportunities for lignocellulosic materials and composites. In: Rowell, R.M., Schultz, T.P., Narayan, R. (eds.) Emerging Technologies for Materials and Chemicals from Biomass, pp. 12–27. American Chemical Society, Washington, DC (1992)
Howard, R.L., Abotsi, E., Jansen, E.L.J., Rensburg Howard, E.J.S.: Lignocellulose biotechnology: issues of bioconversion and enzyme production. African J. Biotechnol. 2, 602–619 (2003). https://doi.org/10.5897/AJB2003.000-1115
Prasad, S., Singh, A., Joshi, H.C.: Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 50, 1–39 (2007). https://doi.org/10.1016/j.resconrec.2006.05.007
McKendry, P.: Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 83, 37–46 (2002). https://doi.org/10.1016/S0960-8524(01)00118-3
Reddy, N., Yang, Y.: Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 23(1), 22–27 (2005)
Ludueña, L., Fasce, D., Alvarez, V.A., Stefani, P.M.: Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources 6, 1440–1453 (2011). https://doi.org/10.15376/biores.6.2.1440-1453
Han, J.S.: Properties of nonwood fibers. In: Proceedings of the Korean Society of Wood Science and Technology Annual Meeting. pp. 3–12 (1998)
Sánchez, C.: Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 27(2), 185–194 (2009)
Gressel, J., Zilberstein, A.: Let them eat (GM) straw. Trends Biotechnol. 21(12), 525–530 (2003)
Kim, M., Day, D.F.: Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 38, 803–807 (2011). https://doi.org/10.1007/s10295-010-0812-8
Li, B.Z., Balan, V., Yuan, Y.J., Dale, B.E.: Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fiber expansion (AFEX) pretreatment. Bioresour. Technol. 101, 1285–1292 (2010). https://doi.org/10.1016/j.biortech.2009.09.044
Sumphanwanich, J., Leepipatpiboon, N., Srinorakutara, T., Akaracharanya, A.: Evaluation of dilute-acid pretreated bagasse, corn cob and rice straw for ethanol fermentation by Saccharomyces cerevisiae. Ann. Microbiol. 58, 219–225 (2008). https://doi.org/10.1007/BF03175320
Van Eylen, D., van Dongen, F., Kabel, M., de Bont, J.: Corn fiber, cobs and stover: enzyme-aided saccharification and co-fermentation after dilute acid pretreatment. Bioresour. Technol. 102, 5995–6004 (2011). https://doi.org/10.1016/j.biortech.2011.02.049
Wu, L., Kumagai, A., Lee, S.H., Endo, T.: Synergistic effect of delignification and treatment with the ionic liquid 1-ethyl-3-methylimidazolium acetate on enzymatic digestibility of poplar wood. Bioresour. Technol. 162, 207–212 (2014). https://doi.org/10.1016/j.biortech.2014.03.144
McMillan, J.D.: Pretreatment of lignocellulosic biomass. Enzymatic conversion of biomass for fuels production. ACS Symp. Ser. 566, 292–324 (1994). https://doi.org/10.1021/bk-1994-0566.ch015
Klinke, H.B., Thomsen, A.B., Ahring, B.K.: Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 66, 10–26 (2004). https://doi.org/10.1007/s00253-004-1642-2
Kahar, P., Taku, K., Tanaka, S.: Enzymatic digestion of corncobs pretreated with low strength of sulfuric acid for bioethanol production. J. Biosci. Bioeng. 110, 453–458 (2010). https://doi.org/10.1016/j.jbiosc.2010.05.002
Wang, G.S., Lee, J.W., Zhu, J.Y., Jeffries, T.W.: Dilute acid pretreatment of corncob for efficient sugar production. Appl. Biochem. Biotechnol. 163, 658–668 (2011). https://doi.org/10.1007/s12010-010-9071-4
Cheng, K.K., Wang, W., Zhang, J.A., Zhao, Q., Li, J.P., Xue, J.W.: Statistical optimization of sulfite pretreatment of corncob residues for high concentration ethanol production. Bioresour. Technol. 102, 3014–3019 (2011). https://doi.org/10.1016/j.biortech.2010.09.117
Lee, J.W., Houtman, C.J., Kim, H.Y., Choi, I.G., Jeffries, T.W.: Scale-up study of oxalic acid pretreatment of agricultural lignocellulosic biomass for the production of bioethanol. Bioresour. Technol. 102, 7451–7456 (2011). https://doi.org/10.1016/j.biortech.2011.05.022
Kumar, A.K., Sharma, S.: Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4, 7 (2017). https://doi.org/10.1186/s40643-017-0137-9
Sahare, P., Singh, R., Laxman, R.S., Rao, M.: Effect of alkali pretreatment on the structural properties and enzymatic hydrolysis of corn cob. Appl. Biochem. Biotechnol. 168, 1806–1819 (2012). https://doi.org/10.1007/s12010-012-9898-y
Wanitwattanarumlug, B., Luengnaruemitchai, A., Wongkasemjit, S.: Characterization of corn cobs from microwave and potassium hydroxide pretreatment. Int. J. Chem. Biol. Eng. 66, 6–10 (2012)
Hromádková, Z., Kováčiková, J., Ebringerová, A.: Study of the classical and ultrasound-assisted extraction of the corn cob xylan. Ind. Crops Prod. 9, 101–109 (1999). https://doi.org/10.1016/S0926-6690(98)00020-X
Zhang, M., Wang, F., Su, R., Qi, W., He, Z.: Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour. Technol. 101, 4959–4964 (2010). https://doi.org/10.1016/j.biortech.2009.11.010
Potumarthi, R., Baadhe, R.R., Jetty, A.: Mixing of acid and base pretreated corncobs for improved production of reducing sugars and reduction in water use during neutralization. Bioresour. Technol. 119, 99–104 (2012). https://doi.org/10.1016/j.biortech.2012.05.103
Loow, Y.L., Wu, T.Y., Tan, K.A., Lim, Y.S., Siow, L.F., Md Jahim, J., Mohammad, A.W., Teoh, W.H.: Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 63(38), 8349–8363 (2015)
**ng, Y., Bu, L., Zheng, T., Liu, S., Jiang, J.: Enhancement of high-solids enzymatic hydrolysis of corncob residues by bisulfite pretreatment for biorefinery. Bioresour. Technol. 221, 461–468 (2016). https://doi.org/10.1016/j.biortech.2016.09.086
Ma, L., Cui, Y., Cai, R., Liu, X., Zhang, C., **ao, D.: Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour. Technol. 180, 1–6 (2015). https://doi.org/10.1016/j.biortech.2014.12.078
Cheng, Y.S., Zheng, Y., Yu, C.W., Dooley, T.M., Jenkins, B.M., Vandergheynst, J.S.: Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 162, 1768–1784 (2010). https://doi.org/10.1007/s12010-010-8958-4
Qing, Q., Zhou, L., Guo, Q., Huang, M., He, Y., Wang, L., Zhang, Y.: A combined sodium phosphate and sodium sulfide pretreatment for enhanced enzymatic digestibility and delignification of corn stover. Bioresour. Technol. (2016). https://doi.org/10.1016/j.biortech.2016.06.063
Sewsynker-Sukai, Y., Gueguim Kana, E.B.: Optimization of a novel sequential alkalic and metal salt pretreatment for enhanced delignification and enzymatic saccharification of corn cobs. Bioresour. Technol. 243, 785–792 (2017). https://doi.org/10.1016/j.biortech.2017.06.175
Merrettig-Bruns, U., Sayder, B.: Pretreatment with ammonia. In: Mussatto, S.I. (ed.) Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, pp. 461–481. Elsevier, Amsterdam (2016)
Cao, N.J., Krishnan, M.S., Du, J.X., Gong, C.S., Ho, N.W.Y., Chen, Z.D., Tsao, G.T.: Ethanol production from corn cob pretreated by the ammonia stee** process using genetically engineered yeast. Biotechnol. Lett. 18, 1013–1018 (1996). https://doi.org/10.1007/BF00129723
Baadhe, R.R., Potumarthi, R., Mekala, N.K.: Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: a comparative study. Bioresour. Technol. 162, 213–217 (2014). https://doi.org/10.1016/j.biortech.2014.03.117
Moulthrop, J.S., Swatloski, R.P., Moyna, G., Rogers, R.D.: High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem. Commun. 40, 1557 (2005). https://doi.org/10.1039/b417745b
Sun, S.N., Li, M.F., Yuan, T.Q., Xu, F., Sun, R.C.: Effect of ionic liquid pretreatment on the structure of hemicelluloses from corncob. J. Agric. Food Chem. 60, 11120–11127 (2012). https://doi.org/10.1021/jf3021464
Chaturvedi, V., Verma, P.: An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. 3, 415–431 (2013). https://doi.org/10.1007/s13205-013-0167-8
García-Cubero, M.T., González-Benito, G., Indacoechea, I., Coca, M., Bolado, S.: Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 100, 1608–1613 (2009). https://doi.org/10.1016/j.biortech.2008.09.012
Chang, V.S., Holtzapple, M.T.: Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 84–86, 5–38 (2000). https://doi.org/10.1385/ABAB:84-86:1-9:5
Ji, G., Gao, C., **ao, W., Han, L.: Mechanical fragmentation of corncob at different plant scales: impact and mechanism on microstructure features and enzymatic hydrolysis. Bioresour. Technol. 205, 159–165 (2016). https://doi.org/10.1016/j.biortech.2016.01.029
Kumakura, M., Kaetsu, I.: Effect of electron beam current on radiation pretreatment of cellulosic wastes with electron beam accelerator. Radiat. Phys. Chem. 23, 523–527 (1984). https://doi.org/10.1016/0146-5724(84)90154-7
Guo, X., Zhang, T., Shu, S., Zheng, W., Gao, M.: Compositional and structural changes of corn cob pretreated by electron beam irradiation. ACS Sustain. Chem. Eng. 5, 420–425 (2017). https://doi.org/10.1021/acssuschemeng.6b01793
Boonsombuti, A., Luengnaruemitchai, A., Wongkasemjit, S.: Enhancement of enzymatic hydrolysis of corncob by microwave-assisted alkali pretreatment and its effect in morphology. Cellulose 20, 1957–1966 (2013). https://doi.org/10.1007/s10570-013-9958-7
Wang, Y., Zhang, J.: A novel hybrid process, enhanced by ultrasonication, for xylan extraction from corncobs and hydrolysis of xylan to xylose by xylanase. J. Food Eng. 77, 140–145 (2006). https://doi.org/10.1016/j.jfoodeng.2005.06.056
Liu, D., Yu, Y., Hayashi, J.I., Moghtaderi, B., Wu, H.: Contribution of dehydration and depolymerization reactions during the fast pyrolysis of various salt-loaded celluloses at low temperatures. Fuel 136, 62–68 (2014). https://doi.org/10.1016/j.fuel.2014.07.025
Zhang, Y., Liu, C.: A new horizon on effects of alkalis metal ions during biomass pyrolysis based on density function theory study. J. Anal. Appl. Pyrolysis 110, 297–304 (2014). https://doi.org/10.1016/j.jaap.2014.09.017
Luque, L., Oudenhoven, S., Westerhof, R., Van Rossum, G., Berruti, F., Kersten, S., Rehmann, L.: Comparison of ethanol production from corn cobs and switchgrass following a pyrolysis-based biorefinery approach. Biotechnol. Biofuels. 9, 1–14 (2016). https://doi.org/10.1186/s13068-016-0661-4
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96(6), 673–686 (2005)
Yang, B., Wyman, C.E.: Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefining 2(1), 26–40 (2008)
Kim, K.H., Hong, J.: Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour. Technol. 77, 139–144 (2001). https://doi.org/10.1016/S0960-8524(00)00147-4
Schacht, C., Zetzl, C., Brunner, G.: From plant materials to ethanol by means of supercritical fluid technology. J. Supercrit. Fluids 46(3), 299–321 (2008)
Zheng, Y., Lin, H.M., Tsao, G.T.: Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol. Prog. 14, 890–896 (1998). https://doi.org/10.1021/bp980087g
Fan, X., Cheng, G., Zhang, H., Li, M., Wang, S., Yuan, Q.: Effects of acid impregnated steam explosion process on xylose recovery and enzymatic conversion of cellulose in corncob. Carbohydr. Polym. 114, 21–26 (2014). https://doi.org/10.1016/j.carbpol.2014.07.051
Zheng, J., Choo, K., Bradt, C., Lehoux, R., Rehmann, L.: Enzymatic hydrolysis of steam exploded corncob residues after pretreatment in a twin-screw extruder. Biotechnol. Rep. 3, 99–107 (2014). https://doi.org/10.1016/j.btre.2014.06.008
Pedraza, L., Flores, A., Toribio, H., Quintero, R., Le Borgne, S., Moss-Acosta, C., Martinez, A.: Sequential thermochemical hydrolysis of corncobs and enzymatic saccharification of the whole slurry followed by fermentation of solubilized sugars to ethanol with the ethanologenic strain Escherichia coli MS04. Bioenergy Res. 9, 1046–1052 (2016). https://doi.org/10.1007/s12155-016-9756-9
Kirk, T.K., Gifford, O., Drive, P., Farrell, R.L.: Enzymatic" combustion": the microbial degradation of lignin. Annu. Rev. Microbiol. 41, 465–505 (1987). https://doi.org/10.1146/annurev.mi.41.100187.002341
Taniguchi, M., Suzuki, H., Watanabe, D., Sakai, K., Hoshino, K., Tanaka, T.: Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 100, 637–643 (2005). https://doi.org/10.1263/jbb.100.637
Lundell, T.K., Mäkelä, M.R., Hildén, K.: Lignin-modifying enzymes in filamentous basidiomycetes - Ecological, functional and phylogenetic review. J. Basic Microbiol 50(1), 5–20 (2010)
Chen, Y., Dong, B., Qin, W., **ao, D.: Xylose and cellulose fractionation from corncob with three different strategies and separate fermentation of them to bioethanol. Bioresour. Technol. 101, 6994–6999 (2010). https://doi.org/10.1016/j.biortech.2010.03.132
Larsson, S., Palmqvist, E., Hahn-Hägerdal, B., Tengborg, C., Stenberg, K., Zacchi, G., Nilvebrant, N.O.: The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 24, 151–159 (1999). https://doi.org/10.1016/S0141-0229(98)00101-X
Chung, I.S., Lee, Y.Y.: Ethanol fermentation of crude acid hydrolyzate of cellulose using high-level yeast inocula. Biotechnol. Bioeng. 27, 308–315 (1985). https://doi.org/10.1002/bit.260270315
Horváth, I.S., Sjöde, A., Alriksson, B., Jönsson, L.J., Nilvebrant, N.O.: Critical conditions for improved fermentability during overliming of acid hydrolysates from spruce. In: Proceedings of the Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology, pp. 1031–1044. (2005)
Qin, L., Li, W.C., Liu, L., Zhu, J.Q., Li, X., Li, B.Z., Yuan, Y.J.: Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol. Biofuels 9, 70 (2016). https://doi.org/10.1186/s13068-016-0485-2
Ando, S., Arai, I., Kiyoto, K., Hanai, S.: Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae. J. Ferment. Technol. 64, 567–570 (1986). https://doi.org/10.1016/0385-6380(86)90084-1
Berlin, A., Balakshin, M., Gilkes, N., Kadla, J., Maximenko, V., Kubo, S., Saddler, J.: Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J. Biotechnol. 125, 198–209 (2006). https://doi.org/10.1016/j.jbiotec.2006.02.021
Pareek, N., Gillgren, T., Jönsson, L.J.: Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour. Technol. 148, 70–77 (2013). https://doi.org/10.1016/j.biortech.2013.08.121
Hsieh, C.W.C., Cannella, D., Jørgensen, H., Felby, C., Thygesen, L.G.: Cellulase inhibition by high concentrations of monosaccharides. J. Agric. Food Chem. 62, 3800–3805 (2014). https://doi.org/10.1021/jf5012962
Holtzapple, M., Cognata, M., Shu, Y., Hendrickson, C.: Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol. Bioeng. 36, 275–287 (1990). https://doi.org/10.1002/bit.260360310
Bezerra, R.M.F., Dias, A.A.: Enzymatic kinetic of cellulose hydrolysis: inhibition by ethanol and cellobiose. Appl. Biochem. Biotechnol. 126, 49–59 (2005). https://doi.org/10.1007/s12010-005-0005-5
Teugjas, H., Väljamäe, P.: Product inhibition of cellulases studied with 14C-labeled cellulose substrates. Biotechnol. Biofuels 6, 104 (2013). https://doi.org/10.1186/1754-6834-6-104
Kumar, R., Wyman, C.E.: Strong cellulase inhibition by Mannan polysaccharides in cellulose conversion to sugars. Biotechnol. Bioeng. 111, 1341–1353 (2014). https://doi.org/10.1002/bit.25218
Kim, Y., **menes, E., Mosier, N.S., Ladisch, M.R.: Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb. Technol. 48, 408–415 (2011). https://doi.org/10.1016/j.enzmictec.2011.01.007
Tejirian, A., Xu, F.: Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme Microb. Technol. 48, 239–247 (2011). https://doi.org/10.1016/j.enzmictec.2010.11.004
Zhu, J., Yong, Q., Xu, Y., Yu, S.: Detoxification of corn stover prehydrolyzate by trialkylamine extraction to improve the ethanol production with Pichia stipitis CBS 5776. Bioresour. Technol. 102, 1663–1668 (2011). https://doi.org/10.1016/j.biortech.2010.09.083
Kim, S.K., Park, D.H., Song, S.H., Wee, Y.J., Jeong, G.T.: Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces cerevisiae. Bioprocess Biosyst. Eng. 36, 659–666 (2013). https://doi.org/10.1007/s00449-013-0888-4
Larsson, S., Reimann, A., Nilvebrant, N.-O., Jönsson, L.J.: Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 77, 91–104 (1999). https://doi.org/10.1385/ABAB:77:1-3:91
Béguin, P., Millet, J., Chauvaux, S., Salamitou, S., Tokatlidis, K., Navas, J., Fu**o, T., Lemaire, M., Raynaud, O., Daniel, M.K.: Bacterial cellulases. Biochem. Soc. Trans. 20, 42–46 (1992). https://doi.org/10.1042/bst0200042
Brar, K.K., Kaur, S., Chadha, B.S.: A novel staggered hybrid SSF approach for efficient conversion of cellulose/hemicellulosic fractions of corncob into ethanol. Renew. Energy. 98, 16–22 (2016). https://doi.org/10.1016/j.renene.2016.03.082
Liu, K., Lin, X., Yue, J., Li, X., Fang, X., Zhu, M., Lin, J., Qu, Y., **ao, L.: High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 101, 4952–4958 (2010). https://doi.org/10.1016/j.biortech.2009.11.013
Itelima, J., Ogbonna, A., Pandukur, S., Egbere, J., Salami, A.: Simultaneous saccharification and fermentation of corn cobs to bio-ethanol by co-culture of aspergillus niger and Saccharomyces cerevisiae. Int. J. Environ. Sci. Dev. 4, 239–242 (2013). https://doi.org/10.7763/IJESD.2013.V4.343
Liang, X., Hua, D., Wang, Z., Zhang, J., Zhao, Y., Xu, H., Li, Y., Gao, M., Zhang, X.: Production of bioethanol using lignocellulosic hydrolysate by the white rot fungus Hohenbuehelia sp. ZW-16. Ann. Microbiol. 63, 719–723 (2013). https://doi.org/10.1007/s13213-012-0524-6
Zhang, M., Shukla, P., Ayyachamy, M., Permaul, K., Singh, S.: Improved bioethanol production through simultaneous saccharification and fermentation of lignocellulosic agricultural wastes by Kluyveromyces marxianus 6556. World J. Microbiol. Biotechnol. 26, 1041–1046 (2010). https://doi.org/10.1007/s11274-009-0267-0
Su, R., Ma, Y., Qi, W., Zhang, M., Wang, F., Du, R., Yang, J., Zhang, M., He, Z.: Ethanol production from high-solid SSCF of alkaline-pretreated corncob using recombinant Zymomonas mobilis CP4. Bioenergy Res. 6, 292–299 (2013). https://doi.org/10.1007/s12155-012-9256-5
Fan, C., Qi, K., **a, X.X., Zhong, J.J.: Efficient ethanol production from corncob residues by repeated fermentation of an adapted yeast. Bioresour. Technol. 136, 309–315 (2013). https://doi.org/10.1016/j.biortech.2013.03.028
Wang, G., Liu, C., Hong, J., Ma, Y., Zhang, K., Huang, X., Zou, S., Zhang, M.: Comparison of process configurations for ethanol production from acid- and alkali-pretreated corncob by Saccharomyces cerevisiae strains with and without β-glucosidase expression. Bioresour. Technol. 142, 154–161 (2013). https://doi.org/10.1016/j.biortech.2013.05.033
Singhania, R.R., Patel, A.K., Sukumaran, R.K., Larroche, C., Pandey, A.: Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Technol. 127, 500–507 (2013). https://doi.org/10.1016/j.biortech.2012.09.012
Acknowledgements
The authors gratefully acknowledge the financial support provided by SERB (Science & Engineering Research Board), INDIA (Grant No. ECR/2017/001038/2017-2020) to carry out this research work.
Funding
All of the sources of funding for the work described in this publication are acknowledged below: We acknowledge the financial support provided by the Research board, INDIA (Grant no. ECR/2017/001038/2017-20-SERB) in accompanying us to complete the work.
Author information
Authors and Affiliations
Contributions
AA: Conceived and designed the analysis, contributed data or analysis tools and wrote the paper. VVM: Wrote the paper. VP: Performed the analysis and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arumugam, A., Malolan, V.V. & Ponnusami, V. Contemporary Pretreatment Strategies for Bioethanol Production from Corncobs: A Comprehensive Review. Waste Biomass Valor 12, 577–612 (2021). https://doi.org/10.1007/s12649-020-00983-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00983-w