Abstract
Purpose
The forage cactus pear (Opuntia spp. and Nopalea spp.) has been sub-utilised, mostly, for ruminant feed during long periods of drought. In this study, Nopalea cochenillifera was investigated as an alternative substrate for the production of xylanases and β-glucosidases by Aspergillus niger and Rhizopus sp.
Methods
The solid-state fermentation of N. cochenillifera by Aspergillus niger and Rhizopus sp. were optimised for xylanases and β-glucosidases production using a central composite face-centered (CCF) experimental design and response surface methodology (RSM), considering the variables water activity, temperature and time. The enzymes obtained were also evaluated for their pH, temperature and storage stability.
Results
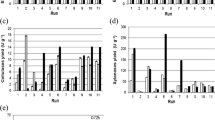
The theoretical optimum cultivation conditions were an initial water activity of 0.865 at 30 °C for 72 h. The equivalent enzymatic activities (U g−1) obtained were 355.56 (A. niger xylanase), 3456.91 (A. niger β-glucosidase), 204.57 (Rhizopus sp. xylanase) and 1630.07 (Rhizopus sp. β-glucosidase). The obtained enzymes presented good stability (~80%) at <60 °C and pH values from 4.0 to 7.0.
Conclusion
The forage cactus is a potential alternative for microbial cultivation instead of synthetic substrates of elevated costs.




Similar content being viewed by others
References
Hendriks, A.T.W.M, Zeeman, G.: Pretreatments to enhance the digestibility of lignocellulosic biomass. Biores. Technol. (2008). doi:10.1016/j.biortech.2008.05.027
Cheirsilpa, B., Kitcha, S.: Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: fed-batch and repeated-batch fermentations. Ind. Crops Prod. (2015). doi:10.1016/j.indcrop.2014.12.035
Prévot, V., Lopez, M., Copinet, E., Duchiron, F.: Comparative performance of commercial and laboratory enzymatic complexes from submerged or solid-state fermentation in lignocellulosic biomass hydrolysis. Biores. Technol. (2013). doi:10.1016/j.biortech.2012.11.135
Soccol, C.R., Vandenberghe, L.P.S.: Overview of applied solid-state fermentation in Brazil. Biochem. Eng. J. (2003). doi:10.1016/S1369-703X(02)00133-X
Waghmare, P.R., Kadam, A.A., Saratale, G.D., Govindwar, S.P.: Enzymatic hydrolysis and characterization of waste lignocellulosic biomass produced after dye bioremediation under solid state fermentation. Biores. Technol. (2014). doi:10.1016/j.biortech.2014.02.099
Baccouche, A., Ennouri, M., Felfoul, I., Attia, H.: A physical stability study of whey-based prickly pear beverages. Food Hydrocol. (2013). doi:10.1016/j.foodhyd.2013.03.007
Medina-Torres, L., La Fuente, E.B.S., Torrestiana-Sanchez, B., Katthain, R.: Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocolloyds (2000). doi:10.1016/S0268-005X(00)00015-1
Barka, N., Abdennouri, M., Makhfouk, M.E., Qourzal, S.: Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. (2013). doi:10.1016/j.jece.2013.04.008
Maran, J.P., Manikandan, S.: Response surface modeling and optimization of process parameters for aqueous extraction of pigments from prickly pear (Opuntia ficus-indica) fruit. Dyes Pigments (2012). doi:10.1016/j.dyepig.2012.06.007
Miller, S.M., Fugate, E.J., Craver, V.O., Smith, J.A., Zimmerman, J.B.: Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol. (2008). doi:10.1021/es7025054
Hassini, L., Bettaieba, E., Desmorieux, H., Torres, S.S., Touil, A.: Desorption isotherms and thermodynamic properties of prickly pear seeds. Ind. Crop. Prod. (2015). doi:10.1016/j.indcrop.2015.01.078
de Castro, J.P., Araújo, E.R., do Rêgo, M.M., do Rêgo, E.R.: In vitro germination and disinfestation of sweet cactus (Nopalea cochenillifera (L.) Salm Dyck). Acta Scient. Agron. (2011). doi:10.4025/actasciagron.v33i3.6275
Dubeux, J.C.B. Jr., dos Santos, M.V.F., Lira, M.A., Santos, D.C., Farias, I., Lima, L.E., Ferreira, R.L.C.: Productivity of Opuntia ficus-indica (L.) Miller under different N and P fertilization and plant population in north-east Brazil. J. Arid. Environ. (2006). doi:10.1016/j.jaridenv.2006.02.015
da Silva, M.G.S., Dubeux, J.C.B. Jr., Assis, L.C.S.L.C., Mota, D.L., da Silva, L.L.S., dos Santos, M.V.F., dos Santos, D.C.: Anatomy of different forage cactus with contrasting insect resistance. J. Arid Environ. (2010). doi:10.1016/j.jaridenv.2009.11.003
Vasconcelos, A.G.V., Lira, M.A., Cavalcanti, V.A.L., Santos, M.V.F., Câmara, T., Willadino, L.: Micropropagação e palma forrageira cv. Miúda (Nopalea cochenillifera - Salm Dyck). Rev. Bras. Cien. Agrar. 2, 28–31 (2007)
Santos, T.C., Reis, N.S., Silva, T.P., Machado, F.P.P., Bonomo, R.C.F., Franco, M.: Prickly palm cactus husk as a raw material for production of ligninolytic enzymes by Aspergills niger. Food Sci. Biotechnol. (2016). doi:10.1007/s10068-016-0
Santos, T.C., Diniz, G.A., de Brito, A.R., Pires, A.J.V., Franco, M.: Effect of solid state fermentation on nutritional content and evaluation of degradability in cactus pear. Rev. Caatinga. (2015). doi:10.1590/1983-21252015v28n328rc
Santos, T.C., Cavalcanti, I.S., Bonomo, R.C.F., Santana, N.B., Franco, M.: Optimization of productions of cellulolytic enzymes by Aspergillus niger using residue of mango a substrate. Ciên. Rur. (2011). doi:10.1590/S0103-84782011005000145
Azevedo, A.M., Rosa, P.A.J., Ferreira, I.F., Aires-Barros, M.R.: Optimisation of aqueous two-phase extraction of human antibodies. J. Biotechnol. (2007). doi:10.1016/j.jbiotec.2007.04.002
Rodrigues, M.I., Iemma, A.F.: Experimental design and process optimization. CRC Press, Boca Raton (2014)
Bailey, M.J., Biely, P., Poutanen, K.: Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. (1992). doi:10.1016/0168-1656(92)90074-J
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. (1959). doi:10.1021/ac60147a030
Gokhale, D.U., Puntambekar, U.S., Deobagkar, D.N.: Xylanase and betaxylosidase production by Aspergillus niger NCIM 1207. Biotechnol. Lett. (1986). doi:10.1007/BF01048472
dos Santos, T.C., Gomes D.P.P., Bonomo R.C.F., Franco, M.: Optimisation of solid state fermentation of potato peel for the production of cellulolytic enzymes. Food Chem. (2012). doi:10.1016/j.foodchem.2011.11.115
Handa, C.L., Couto, U.R., Vicensoti, A.H., Georgetti, S.R., Ida, E.I.: Optimisation of soy flour fermentation parameters to produce b-glucosidase for bioconversion into aglycones. Food Chem. (2014). doi:10.1016/j.foodchem.2013.11.101
Matsuura, M., Sasaki, J., Murao, S.: Studies on b-glucosidases from soybeans that hydrolyse daidzin and genistin: Isolation and characterization of an isozyme. Biosci. Biotechnol. Biochem. (1995).doi:10.1271/bbb.59.1623
Alva, S., Anupama, J., Savla, J., Chiu, Y.Y., Vyshali, P., Shruti, M.: Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI 12 in solid state culture. Afr. J. Biotechnol. 6, 576–581 (2007)
Biazus, J.P.M., Souza, R.R., Santana, J.C.C., Tambourgi, E.B.: Otimização da secagem do malte de Zea mays. Cien Tecnol. Alim. (2006). doi:10.1590/S0101-20612006000400012
Omemu, A.M., Akpan, I., Bankole, M.O., Teniola, O.D.: Hydrolysis of raw tuber starches by amylase of Aspergillus niger AM07 isolated from the soil. Afr. J. Biotechnol. 4, 19–25 (2005)
dos Santos, T.C., Filho, G.A., Oliveira, A.C.O., Rocha, T.J.O., Machado, F.P.P., Bonomo, R.C.F., Mota, K.I.A., Franco, M.: Application of response surface methodology for producing cellulolytic enzymes by solid-state fermentation from the puple mombin (Spondias purpurea L.) residue. Food Sci. Biotechnol. (2013). doi:10.1007/s10068-013-0001-4
Shafique, S., Bajwa, R., Shafique, S.: Screening of Aspergillus niger and A. flavus strains for extra cellular alpha-amylase activity. Pak J. Bot. 41, 897–905 (2009)
Abdeshahian, P., Samat, N., Hamid, A.A., Yusoff, W.M.W.: Solid substrate fermentation for cellulase production using palm kernel cake as a renewable lignocellulosic source in packed-bed bioreactor. Biotechnol Biopr. Eng. (2011). doi:10.1007/s12257-010-0320-8
Zimbardi, A.L.R.L., Sehn, C., Meleiro, L.P., Souza, F.H.M., Masui, D.C., Nozawa, M.S.F., Guimarães, L.H.S., Jorge, J.A.J., Furriel, R.P.M. Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol. Sci. (2013). doi:10.3390/ijms14022875
Saha, S.P., Ghosh, S.: Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatal. Agricult. Biotechnol. (2014). doi:10.1016/j.bcab.2014.03.003
Kaushik, P., Mishra, A., Malik, A.: Dual application of agricultural residues for xylanase production and dye removal through solid state fermentation. Int. Biodeter. Biodegrd. (2014). doi:10.1016/j.ibiod.2014.08.006
Alves-Prado, H.F., Pavezzi, F.C., Leite RSR, de Oliveira, V.M., Sette, L.D., da Silva, R.: Screening and production study of microbial xylanase producers from brazilian cerrado. Appl. Biochem. Biotechnol. (2010). doi:10.1007/s12010-009-8823-5
Garcia, N.F.L., Santos, F.R.S., Gonçalves, F.A., da Paz, M.F., Fonseca, G.G., Leite, R.S.R.: Production ofβ -glucosidase on solid-state fermentation byLichtheimia ramosain agroindustrial residues: characterization and catalytic properties of the enzymatic extract. Electr. J. Biotechnol. (2015). doi:10.1016/j.ejbt.2015.05.007
Sharma, R., Rawat, R., Bhogal, R.S., Oberoi, H.S.: Multi-component thermostable cellulolytic enzyme production byAspergillus nigerHN-1 using pea pod waste: appraisal of hydrolytic potential with lignocellulosic biomass. Process Biochem. (2015). doi:10.1016/j.procbio.2015.01.025
Scholl, A.l., Menegol, D., Pitarelo, A.P., Fontana, R.C., Zandoná Filho, A., Ramos, L.P., Dillon, A.J.P., Camassola, M.: Elephant grass (Pennisetum purpureum Schum.) pretreated via steam explosion as a carbon source for cellulases and xylanases in submerged cultivation. Ind. Crops Prod. (2015). doi:10.1016/j.indcrop.2015.03.056
Shah, A.R., Madamwar, D.: Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem. (2005). doi:10.1016/j.procbio.2004.06.041
Bai, H., Wang, H., Sun, J., Irfan, M., Han, M., Huang, Y., Han, X., Yang, Q.: Production, purification and characterization of novel beta glucosidase from newly isolated Penicilium simplicissimum H-11 in submerged fermentation. EXCLI. J. (2013). doi:10.17877/DE290R-7346
Gao, L., Gao, F., Jiang, X., Zhang, C., Zhang, D., Wang, L., Wu, G., Chen, S.: Biochemical characterization of a new β-glucosidase (Cel3E) from Penicillium piceum and its application in boosting lignocelluloses bioconversion and forming disaccharide inducers: New insights into the role of β-glucosidase. Process Biochem. (2014). doi:10.1016/j.procbio.2014.02.012
Langston, J., Sheehy, N., Xu, F.: Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochim Biophys Acta. (2006). doi:10.1016/j.bbapap.2006.03.009
Tiwari, P., Misra, B.N., Sangwan, N.S.: β-Glucosidases from the fungus trichoderma: an efficient cellulase machinery in biotechnological applications. BioMed Res. Int. (2013). doi:10.1155/2013/203735
Riou, C., Salmon, J.M., Vallier, M.J., Günata, Z., Barre, P.: Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 64, 3607–3614 (1998)
Jäger, S., Brumbauer, A., Fehér, E., Réczey, K., Kiss, L.: Production and characterization of β-glucosidases from diferent Aspergillus strains. World J. Microb. Biotechnol. (2001). doi:10.1023/A:1011948405581
Acknowledgements
Authors would like to acknowledge the Banco do Nordeste do Brasil (BNB, Brazil), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for their important financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos, T.C., dos Santos Reis, N., Silva, T.P. et al. Production, Optimisation and Partial Characterisation of Enzymes from Filamentous Fungi Using Dried Forage Cactus Pear as Substrate. Waste Biomass Valor 9, 571–579 (2018). https://doi.org/10.1007/s12649-016-9810-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9810-z