Abstract
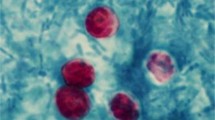
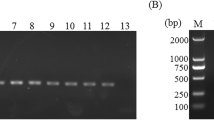
The comparative efficacies of different conventional parasitological methods and nested PCR for diagnosis of bovine cryptosporidiosis in faecal samples were evaluated. Among the 100 samples collected from calves in and around Ludhiana Direct faecal smear staining technique revealed 25.0 % positivity for the oocysts of Cryptosporidium spp. with sensitivity and specificity of 68.12 and 92.98 %, respectively. Zinc sulphate solution floatation and saturated sugar solution floatation staining techniques showed sensitivity and specificity of 83.92 and 96.36; 81.03 and 98.14 %, respectively. Products of the primary PCR of Cryptosporidium spp. directed against small subunit (18S) ribosomal RNA when employed as template in nested PCR produced the amplicons of desired size (834 bp) in 47.0 % of the samples. Amplification of 834 bp fragment was also observed in positive control, while no amplification was observed in negative control. Results indicated PCR assays as highly sensitive and specific techniques for the screening of the samples for Cryptosporidium spp. but in develo** countries and under field conditions where limited resources do not allow the application of PCR assays, concentration staining methods are recommended.



Similar content being viewed by others
References
Bakheit MA, Torra D, Palomino LA, Thekisoe OM, Mbati PA, Ongerth J, Karanis P (2008) Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet Parasitol 158:11–22
Barwick RS, Mohammed HO, White AB, Bryan RT (2000) Detection of Cryptosporidium parvum and Cryptosporidium muris in soil samples. Biol Fertil Soils 31:385–390
Casemore DP, Wright SE, Coop RL (1997) Cryptosporidiosis-human and animal epidemiology. In: Fayer R (ed) Cryptosporidium and cryptosporidiosis. CRC, Boca Raton, pp 65–92
Current WL, Reese NC, Ernst JV, Bailey WS, Heyman MB, Weinstein WM (1983) Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies on outbreak and experimental transmission. N Engl J Med 308:1252–1258
Fayer R, Speer CA, Dubey JP (1997) The general biology of Cryptosporidium. In: Fayer R (ed) Cryptosporidium and cryptosporidiosis. CRC, Boca raton, pp 1–42
Henriksen SA, Krogh HV (1985) Bovine cryptosporidiosis in Denmark. Prevalence, age, distribution and seasonal variation. Nord Vet Med 37:34–41
Kar S, Gawlowska S, Daugschies A, Bangoura B (2011) Quantitative comparison of different purification and detection methods for Cryptosporidium parvum oocysts. Vet Parasitol 177:366–370
Kaushik K, Khurana S, Wanchu A, Malla N (2008) Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Acta Trop 107:1–7
Leek RG, Fayer R (1984) Prevalence of Cryptosporidium infections and their relation to diarrhea in calves on 12 dairy farms in Glicia (NW Spain). Vet Parasitol 106:1–10
MacPherson DW, McQueen R (1993) Cryptosporidiosis: multi-attribute evaluation of six diagnostic methods. J Clin Microbiol 31:198–202
Morgan UM, Pallant L, Dwyer D, Forbes DA, Rich G, Thompson RCA (1998) Comparison of PCR and microscopy for the detection of Cryptosporidium parvum in human faecal samples: clinical trial. J Clin Microbiol 36:995–998
OIE (2008) Cryptosporidiosis. Terr Man. Chapter 2.9.4
Paul S, Chandra D, Tewari AK, Banerjee PS, Ray DD, Boral R, Rao JR (2009) Comparative evaluation and economic assessment of coprological diagnostic methods and PCR for detection of Cryptosporidium spp. in bovines. Vet Parasitol 164:291–295
Singh BB, Sharma R, Kumar H, Banga HS, Aulakh RS, Gill JPS, Sharma JK (2006) Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet Parasitol 140:162–165
Skotarczak B (2009) Methods for parasitic protozoan detection in the environmental samples. Parasite 16:183–190
Webster KA, Smith HV, Giles M, Dawson L, Robertson LJ (1996) Detection of Cryptosporidium oocysts in faeces: comparison of conventional coproscopical methods and polymerase chain reaction. Vet Parasitol 61:5–13
**ao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Monsali RJ, Fayer R, Lal AA (1999) Phylogenetic analysis of Cryptosporidium parasites based on the SSU rRNA gene locus. App Environ Microbiol 65:1578–1583
**ao L, Alderisio KA, Jiang J (2006) Detection of Cryptosporidium oocysts in water: effect of the number of samples and analytic replicates on test results. Appl Environ Microbiol 72(9):5942–5947
Acknowledgments
Authors are highly thankful to Director of Research, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India for providing all necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhat, S.A., Dixit, M., Juyal, P.D. et al. Comparison of nested PCR and microscopy for the detection of cryptosporidiosis in bovine calves. J Parasit Dis 38, 101–105 (2014). https://doi.org/10.1007/s12639-012-0201-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-012-0201-5