Abstract
Purpose
Among the myriad of abiotic stresses, drought is a prominent stress that harms plant growth, development, crop yield, and quality. Nowadays, the ideas of sustainable agriculture have focused on the introduction of biologically synthesized nanomaterials to maximize crop yield with the least amount of potential toxic effects. The application of nanoparticles is thought to be one of the most effective and promising methods for reducing the consequences of drought stress on crops.
Methods
The Si nanoparticles were characterized in detail and four different concentrations of Si NPs (30 ppm, 60 ppm, 90 ppm, and 120 ppm) were used for the study at two irrigation regimes with 100% soil moisture content (SMC) under well-watered and 50% SMC under drought stress conditions.
Results
Substantial improvement in almost all physiological parameters was observed after foliar application of Si NPs at all concentrations, but 60 ppm concentration was found to be the most effective in improving overall plant resistance to drought stress. This was evident by lowered hydrogen peroxidase (H2O2) and lipid peroxidation (MDA) content and increased relative water content (RWC), antioxidant enzyme activities (APOX, CAT, and SOD), chlorophyll content, and proline content. A total of 5 stress-related genes (DREB2, MYB33 MYB3R, WRKY 19, and SnRK 2.4) were studied, which were found to be upregulated after application of Si NPs, indicating its stimulatory role even at molecular levels.
Conclusion
Our detailed investigational study and findings will open a pragmatic option in research studies related to usage of nanoparticles in mitigating of drought tolerance of wheat. The study can definitely be used a reference for future work in diverse crops, where production is being drastically compromised due to climate change, particularly water deficiency.
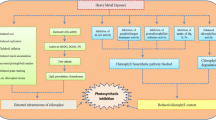
Graphical abstract
Illustrating role of Si NPs in drought stress mitigation (created in BioRender.com)

Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article. Supplementary data (if any) can be made available from the corresponding author on reasonable request.
References
Mathur S, Raikalal P, Jajoo A (2019) Physiological responses of wheat to environmental stresses. In: Wheat Production in Changing Environments. Springer, pp 31–61
Shewry PR, Hey SJ (2015) The contribution of wheat to human diet and health. Food Energy Secur 4:178–202
Hatfield JL, Dold C (2019) Water-use efficiency: advances and challenges in a changing climate. Front Plant Sci 10:103
Pandey A, Khobra R, Mamrutha HM et al (2022) Elucidating the drought responsiveness in wheat genotypes. Sustainability 14:3957
Wang N, Zhang W, Qin M et al (2017) Drought tolerance conferred in soybean (Glycine max. L) by GmMYB84, a novel R2R3-MYB transcription factor. Plant Cell Physiol 58:1764–1776
Kumari A, Kaur R, Kaur R (2018) An insight into drought stress and signal transduction of abscisic acid. Plant Sci Today 5:72–80
Chanu TT, Upadhyaya H (2019) Zinc oxide nanoparticle-induced responses on plants: a physiological perspective. In: Nanomaterials in plants, algae and microorganisms. Elsevier, pp 43–64
Ahmad Z, Tahseen S, Wasi A et al (2022) Nanotechnological interventions in agriculture. Nanomaterials 12:2667
Joudeh N, Linke D (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnology 20:1–29
Zulfiqar F, Ashraf M (2021) Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol Biochem 160:257–268
Dhiman P, Rajora N, Bhardwaj S et al (2021) Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol Biochem 162:110–123. https://doi.org/10.1016/j.plaphy.2021.02.023
Wang M, Wang R, Mur LAJ et al (2021) Functions of silicon in plant drought stress responses. Hortic Res 8:254
Zargar SM, Mahajan R, Bhat JA et al (2019) Role of silicon in plant stress tolerance: opportunities to achieve a sustainable crop** system. 3 Biotech 9:73. https://doi.org/10.1007/s13205-019-1613-z
Bokor B, Soukup M, Vaculík M et al (2019) Silicon uptake and localisation in date palm (Phoenix dactylifera)–A unique association with sclerenchyma. Front Plant Sci 10:988
Kumar S, Soukup M, Elbaum R (2017) Silicification in grasses: variation between different cell types. Front Plant Sci 8:438
Khan ZS, Rizwan M, Hafeez M et al (2020) Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ Sci Pollut Res 27:4958–4968
Wang L, Ning C, Pan T, Cai K (2022) Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int J Mol Sci 23:1947
Mostofa MG, Rahman MM, Ansary MMU et al (2021) Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit Rev Biotechnol 41:918–934. https://doi.org/10.1080/07388551.2021.1892582
Rizwan M, Ali S, Malik S et al (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41:1–12
Sutulienė R, Ragelienė L, Samuolienė G et al (2021) The response of antioxidant system of drought-stressed green pea (Pisum sativum L.) affected by watering and foliar spray with silica nanoparticles. Horticulturae 8:35
Behboudi F, TahmasebiSarvestani Z, Kassaee MZ et al (2018) Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J Agric Sci Technol 20:1479–1492
Tripathi DK, Singh VP, Prasad SM et al (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198
Tripathi DK, Singh S, Singh VP et al (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81
Jabeen N, Maqbool Q, Sajjad S et al (2017) Biosynthesis and characterisation of nano- silica as potential system for carrying streptomycin at nano-scale drug delivery. IET Nanobiotechnol 11(5):557–561. https://doi.org/10.1049/iet-nbt.2016.0106
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Kono Y (1978) Generation of superoxide radicals during auto-oxidation of hydroxyl-amine hydrochloride an assay for SOD. Arch Biochem Biophys 186:189–195
Aebi HE (1983) [13] Catalase in vitro. Methods Enzym Anal 105:121–126
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant cell Physiol 22:867–880
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Wei L, Wang L, Yang Y et al (2015) Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front Plant Sci 6:458
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Rahimzadeh CY, Barzinjy AA, Mohammed AS, Hamad SM (2022) Green synthesis of SiO2 nanoparticles from Rhus coriaria L. extract: Comparison with chemically synthesized SiO2 nanoparticles. PLoS One 17:e0268184
Periakaruppan R, Danaraj J (2022) Biosynthesis of silica nanoparticles using the leaf extract of Punica granatum and assessment of its antibacterial activities against human pathogens. Appl Biochem Biotechnol 194:5594–5605
Al-Azawi MT, Hadi SM, Mohammed CH (2019) Synthesis of silica nanoparticles via green approach by using hot aqueous extract of Thuja orientalis leaf and their effect on biofilm formation. Iraqi J Agric Sci 50:245–255
Sun J, Xu Z, Li W, Shen X (2017) Effect of nano-SiO2 on the early hydration of alite-sulphoaluminate cement. Nanomaterials 7:102
Tabatabaei S, Shukohfar A, Aghababazadeh R, Mirhabibi A (2006) Experimental study of the synthesis and characterisation of silica nanoparticles via the sol-gel method. J Phys Conf Ser 26:371–374. https://doi.org/10.1088/1742-6596/26/1/090
Lu Z, Wang J, Li Q et al (2009) Controllable synthesis of nanosilica surface-grafted PMMA macromonomers via catalytic chain transfer polymerization. Eur Polym J 45:1072–1079
Shokri B, Firouzjah MA, Hosseini SI (2009) FTIR analysis of silicon dioxide thin film deposited by metal organic-based PECVD. In: Proceedings of 19th international symposium on plasma chemistry society. pp 26–31
Verma J (2018) Analysis on synthesis of silica nanoparticles and its effect on growth of T. harzianum & rhizoctonia species. Biomed J Sci Tech Res 10:7890–7897. https://doi.org/10.26717/bjstr.2018.10.001972
Panwar K, Jassal M, Agrawal AK (2015) In situ synthesis of Ag–SiO2 Janus particles with epoxy functionality for textile applications. Particuology 19:107–112
Babu RH, Yugandhar P, Savithramma N (2018) Synthesis, characterization and antimicrobial studies of bio silica nanoparticles prepared from Cynodon dactylon L.: a green approach. Bull Mater Sci 41:1–8
Khan MT, Ahmed S, Shah AA et al (2021) Influence of zinc oxide nanoparticles to regulate the antioxidants enzymes, some osmolytes and agronomic attributes in Coriandrum sativum L. grown under water stress. Agronomy 11:2004
Hussain S, Rao MJ, Anjum MA, et al (2019) Oxidative stress and antioxidant defense in plants under drought conditions. Plant Abiotic Stress Toler Agron Mol Biotechnol Approaches 207–219
Ahanger MA, Tomar NS, Tittal M et al (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744
Sohag AAM, Tahjib-Ul-Arif M, Brestic M et al (2020) Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant, Soil Environ 66:7–13
Abid M, Ali S, Qi LK et al (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep 8(1):4615
Noctor G, Reichheld J-P, Foyer CH (2018) ROS-related redox regulation and signaling in plants. In: Seminars in cell & developmental biology. Elsevier, pp 3–12
Antonić DD, Subotić AR, Dragićević MB et al (2020) Effects of exogenous salicylic acid on drought response and characterization of dehydrins in Impatiens walleriana. Plants 9:1589
Bayati P, Karimmojeni H, Razmjoo J et al (2022) Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 8:193
Xu J, Guo L, Liu L (2022) Exogenous silicon alleviates drought stress in maize by improving growth, photosynthetic and antioxidant metabolism. Environ Exp Bot 201:104974
World Agricultural Production. United States Department of Agriculture. Circular Series WAP 11–22 November 2022. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf. Accessed on 30 November 2022
Adrees M, Khan ZS, Rizwan M, Ali S (2022) Foliar spray of silicon nanoparticles improved the growth and minimized cadmium (Cd) in wheat under combined Cd and water-limited stress. Environ Sci Pollut Res 29(51):77321–77332
Akhtar N, Ilyas N (2022) Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 477(1–2):99–115
Mukarram M, Khan MMA, Corpas FJ (2021) Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J Hazard Mater 412:125254
Khan MT, Ahmed S, Shah AA (2022) Regulatory role of folic acid in biomass production and physiological activities of Coriandrum sativum L. under irrigation regimes. Int J Phytoremediation 24:1025–1038
Elshayb OM, Nada AM, Ibrahim HM et al (2021) Application of silica nanoparticles for improving growth, yield, and enzymatic antioxidant for the hybrid rice EHR1 growing under water regime conditions. Materials 14(5):1150. https://doi.org/10.3390/ma14051150
Yang X, Lu M, Wang Y et al (2021) Response mechanism of plants to drought stress. Horticulturae 7:50
Aarti PD, Tanaka R, Tanaka A (2006) Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol Plant 128:186–197
Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F (1999) Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091–1100
Mafakheri A, Siosemardeh AF, Bahramnejad B et al (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Parveen A, Liu W, Hussain S et al (2019) Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 8:431
Helaly MN, El-Hoseiny H, El-Sheery NI et al (2017) Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol Biochem 118:31–44
Cao B, Ma Q, Zhao Q et al (2015) Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci Hortic (Amsterdam) 194:53–62
Hussain HA, Men S, Hussain S et al (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9:1–12
Naikwade PV (2023) Plant responses to drought stress: morphological, physiological, molecular approaches, and drought resistance. In: Plant Metabolites under Environmental Stress. Apple Academic Press, pp 149–183
Zahedi SM, Moharrami F, Sarikhani S, Padervand M (2020) Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci Rep 10:1–18
Gahlaut V, Jaiswal V, Kumar A, Gupta PK (2016) Transcription factors involved in drought tolerance and their possible role in develo** drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.). Theor Appl Genet 129:2019–2042
Singh D, Laxmi A (2015) Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Front Plant Sci 6:895
Qin Y, Wang M, Tian Y (2012) Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Mol Biol Rep 39:7183–7192. https://doi.org/10.1007/s11033-012-1550-y
Cai H, Tian S, Liu C, Dong H (2011) Identification of a MYB3R gene involved in drought, salt and cold stress in wheat (Triticum aestivum L.). Gene 485:146–152
Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15:247–258
Niu C, Wei W, Zhou Q et al (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35:1156–1170
Yalda S, Sadati R, Godehkahriz SJ et al (2022) Zinc oxide nanoparticles enhance drought tolerance in wheat via physio-biochemical changes and stress genes expression. Iran J Biotechnol 20(1):e3027. https://doi.org/10.30498/ijb.2021.280711.3027
Feng J, Wang L, Wu Y et al (2019) TaSnRK2. 9, a sucrose non-fermenting 1-related protein kinase gene, positively regulates plant response to drought and salt stress in transgenic tobacco. Front Plant Sci 9:2003
Acknowledgements
We acknowledge the financial support provided to us in the form of university minor project by Guru Jambheshwar University of Science & Technology, Hisar, Haryana, India and the research facilities of All India Institute of Medical Sciences (AIIMS) for conducting SEM/TEM Analysis.
Funding
The work was partially supported by the financial grant received in the form of University Minor Project (Guru Jambheshwar University of Science & Technology, Hisar, Haryana, India) via letter no Endst No: DR/2022/07 dated 10/02/2022.
Author information
Authors and Affiliations
Contributions
Sapna Grewal (SG), Rekha Boora (RB), Promila Sheoran (PS), Neelam Rani (NR), Santosh Kumari (SK), Rajesh Thakur (RT). SG -conceived and designed the research work. RB -conducted the experiments and did the research work. PS, RB, NR -data collection and analysis. SG, RB SK, RT- Contributed to data interpretation and manuscript preparation. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors report no conflict of interest.
Competing Interests
The authors declare no competing interests.
Ethics Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boora, R., Sheoran, P., Rani, N. et al. Biosynthesized Silica Nanoparticles (Si NPs) Helps in Mitigating Drought Stress in Wheat Through Physiological Changes and Upregulation of Stress Genes. Silicon 15, 5565–5577 (2023). https://doi.org/10.1007/s12633-023-02439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02439-x