Abstract
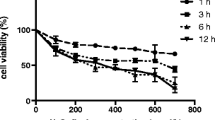
The aim of the present study was to investigate the in vitro antioxidant potential of Bacillus coagulans T242. B. coagulans T242 showed better antioxidant activities, including the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging ability, lipid peroxidation inhibiting ability and reducing ability, than those exerted by Lactobacillus rhamnosus GG (LGG). B. coagulans T242 positively regulated the expression of the nuclear factor erythroid 2-relatedfactor 2/Kelch-like ECH-associated protein-1 (Nrf2/Keap1) pathway-related proteins (Nrf2, Keap1, heine oxygenase-1 (HO-1)); increased antioxidant enzymes (glutathione peroxidase (GSH-Px), catalase (CAT), superoxide dismutase (SOD)); reduced the content of malondialdehyde (MDA) level; decreased the expression of inflammatory-related cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α); and thus increased the survival rate in 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH)-damaged HT-29 cells. This study proved that B. coagulans T242 exerted antioxidative effects by quenching oxygen free radicals and activating the Nrf2 signaling pathway in HT-29 cells.






Similar content being viewed by others
Data Availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang L, Ding L, Yu Z, Zhang T, Ma S, Liu J (2016) Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res Int 90:33–41. https://doi.org/10.1016/j.foodres.2016.10.023
Ji K, Cho YS, Kim YT (2018) Tyrosinase inhibitory and anti-oxidative effects of lactic acid bacteria isolated from dairy cow feces. Probiotics Antimicrob Proteins 10(1):43–55. https://doi.org/10.1007/s12602-017-9274-x
Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L (2018) Role of ROS and nutritional antioxidants in human diseases. Front Physiol 9:477. https://doi.org/10.3389/fphys.2018.00477
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W (2016) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016:4350965. https://doi.org/10.1155/2016/4350965
Cabello-Verrugio C, Vilos C, Rodrigues-Diez R, Estrada L (2018) Oxidative stress in disease and aging: mechanisms and therapies 2018. Oxid Med Cell Longev 2018:2835189. https://doi.org/10.1155/2018/2835189
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10(1):9–17. https://doi.org/10.1038/nchembio.1416
Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta 1792(7):643–650. https://doi.org/10.1016/j.bbadis.2008.12.006
Yu X, Li Y, Wu Q, Shah NP, Wei H, Xu F (2020) Genomic analysis for antioxidant property of Lactobacillus plantarum FLPL05 from Chinese longevity people. Probiotics Antimicrob Proteins 12(4):1451–1458. https://doi.org/10.1007/s12602-020-09704-0
Finamore A, Ambra R, Nobili F, Garaguso I, Raguzzini A, Serafini M (2018) Redox Role of Lactobacillus casei Shirota Against the Cellular Damage Induced by 2,2’-Azobis (2-Amidinopropane) Dihydrochloride-Induced Oxidative and Inflammatory Stress in Enterocytes-Like Epithelial Cells. Front Immunol 9:1131. https://doi.org/10.3389/fimmu.2018.01131
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63(14):3615–3626. https://doi.org/10.1021/jf506326t
Elsayed Azab A, Adwas A, Ibrahim Elsayed AS, Adwas A, Ibrahim Elsayed AS, Quwaydir FA (2019) Oxidative stress and antioxidant mechanisms in human body. J Biotechnol Bioeng 6(1):43–47. https://doi.org/10.15406/jabb.2019.06.00173
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9(5). https://doi.org/10.3390/nu9050521
Rajoka MSR, Mehwish HM, Hayat HF, Hussain N, Sarwar S, Aslam H, Nadeem A, Shi J (2019) Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob Proteins 11(4):1132–1142. https://doi.org/10.1007/s12602-018-9494-8
Mu G, Gao Y, Tuo Y, Li H, Zhang Y, Qian F, Jiang S (2018) Assessing and comparing antioxidant activities of lactobacilli strains by using different chemical and cellular antioxidant methods. J Dairy Sci 101(12):10792–10806. https://doi.org/10.3168/jds.2018-14989
Persichetti E, De Michele A, Codini M, Traina G (2014) Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 30(7–8):936–938. https://doi.org/10.1016/j.nut.2013.12.009
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62(4):1097–1103. https://doi.org/10.1007/s00284-010-9827-7
Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y (2014) Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas J Anim Sci 27(8):1131–1140. https://doi.org/10.5713/ajas.2013.13737
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29(4):813–835. https://doi.org/10.1016/j.femsre.2004.12.001
Safronova LS, Skorochod IA, Ilyash VM (2021) Antioxidant and antiradical properties of probiotic strains Bacillus amyloliquefaciens ssp. plantarum. Probiotics Antimicrob Proteins 13 (6):1585–1597. https://doi.org/10.1007/s12602-021-09827-y
Jemil N, Ben Ayed H, Manresa A, Nasri M, Hmidet N (2017) Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol 17(1):144. https://doi.org/10.1186/s12866-017-1050-2
Majlesi M, Shekarforoush SS, Ghaisari HR, Nazifi S, Sajedianfard J, Eskandari MH (2017) Effect of Probiotic Bacillus Coagulans and Lactobacillus Plantarum on alleviation of mercury toxicity in rat. Probiotics Antimicrob Proteins 9(3):300–309. https://doi.org/10.1007/s12602-016-9250-x
Li LY, Lei K, Xu X, Rajput IR, Yu DY, Li WF (2013) Protective effect of Bacillus subtilis B10 against hydrogen peroxide-induced oxidative stress in a murine macrophage cell line. Int J Agric Biol 15(5)
Wang Y, Wu Y, Wang Y, Fu A, Gong L, Li W, Li Y (2017) Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl Microbiol Biotechnol 101(7):3015–3026. https://doi.org/10.1007/s00253-016-8032-4
Kobatake E, Nakagawa H, Seki T, Miyazaki T (2017) Protective effects and functional mechanisms of Lactobacillus gasseri SBT2055 against oxidative stress. PLoS ONE 12(5):e0177106. https://doi.org/10.1371/journal.pone.0177106
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28(2):214–220. https://doi.org/10.1016/j.fm.2010.03.007
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490. https://doi.org/10.3389/fmicb.2017.01490
Sui L, Zhu X, Wu D, Ma T, Tuo Y, Jiang S, Qian F, Mu G (2020) In vitro assessment of probiotic and functional properties of Bacillus coagulans T242. Food Biosci 36. https://doi.org/10.1016/j.fbio.2020.100675
Li C, Huang Q, Fu X, Yue XJ, Liu RH, You LJ (2015) Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int J Biol Macromol 75:298–305. https://doi.org/10.1016/j.ijbiomac.2015.01.010
Tang W, **ng Z, Li C, Wang J, Wang Y (2017) Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem 221:1642–1649. https://doi.org/10.1016/j.foodchem.2016.10.124
Stinco CM, Baroni MV, Di Paola Naranjo RD, Wunderlin DA, Heredia FJ, Meléndez-Martínez AJ, Vicario IM (2015) Hydrophilic antioxidant compounds in orange juice from different fruit cultivars: Composition and antioxidant activity evaluated by chemical and cellular based (Saccharomyces cerevisiae) assays. J Food Compos Anal 37:1–10. https://doi.org/10.1016/j.jfca.2014.09.006
Felice DL, Sun J, Liu RH (2009) A modified methylene blue assay for accurate cell counting. Journal of Functional Foods 1(1):109–118. https://doi.org/10.1016/j.jff.2008.09.014
Mu G, Li H, Tuo Y, Gao Y, Zhang Y (2019) Antioxidative effect of Lactobacillus plantarum Y44 on 2,2’-azobis(2-methylpropionamidine) dihydrochloride (ABAP)-damaged Caco-2 cells. J Dairy Sci 102(8):6863–6875. https://doi.org/10.3168/jds.2019-16447
Zainodini N, Hassanshahi G, Hajizadeh M, Khanamani Falahati-Pour S, Mahmoodi M, Mirzaei MR (2018) Nisin induces cytotoxicity and apoptosis in human asterocytoma cell line (SW1088). Asian Pac J Cancer Prev 19(8):2217–2222. https://doi.org/10.22034/APJCP.2018.19.8.2217
De Giani A, Bovio F, Forcella M, Fusi P, Sello G, Di Gennaro P (2019) Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 9(1):88. https://doi.org/10.1186/s13568-019-0813-6
Molska M, Regula J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 11(10). https://doi.org/10.3390/nu11102453
Park JM, Lee JS, Song JE, Sim YC, Ha SJ, Hong EK (2015) Cytoprotective effect of hispidin against palmitate-induced lipotoxicity in C2C12 myotubes. Molecules 20(4):5456–5467. https://doi.org/10.3390/molecules20045456
Jaramillo MC, Zhang DD (2013) The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27(20):2179–2191. https://doi.org/10.1101/gad.225680.113
Yang Y, Tian Z, Ding Y, Li X, Zhang Z, Yang L, Zhao F, Ren F, Guo R (2018) EGFR-targeted immunotoxin exerts antitumor effects on esophageal cancers by increasing ROS accumulation and inducing apoptosis via inhibition of the Nrf2-Keap1 pathway. J Immunol Res 2018:1090287. https://doi.org/10.1155/2018/1090287
Saw CLL, Wu Q, Kong AN (2010) Anti-cancer and potential chemopreventive actions of ginseng by activating Nrf2 (NFE2L2) anti-oxidative stress/anti-inflammatory pathways. Chin Med-UK 5(1):1–7. https://doi.org/10.1186/1749-8546-5-37
Jiang CS, Zhuang CL, Zhu K, Zhang J, Muehlmann LA, Figueiro Longo JP, Azevedo RB, Zhang W, Meng N, Zhang H (2018) Identification of a novel small-molecule Keap1-Nrf2 PPI inhibitor with cytoprotective effects on LPS-induced cardiomyopathy. J Enzyme Inhib Med Chem 33(1):833–841. https://doi.org/10.1080/14756366.2018.1461856
Paloczi J, Varga ZV, Hasko G, Pacher P (2018) Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid Redox Signal 29(1):75–108. https://doi.org/10.1089/ars.2017.7144
Wu J, **a S, Kalionis B, Wan W, Sun T (2014) The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int 2014:615312. https://doi.org/10.1155/2014/615312
Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97(2):809–817. https://doi.org/10.1007/s00253-012-4241-7
Gu SB, Zhao LN, Wu Y, Li SC, Sun JR, Huang JF, Li DD (2015) Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World J Microbiol Biotechnol 31(6):851–863. https://doi.org/10.1007/s11274-015-1838-x
Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, Paik HD (2019) Antagonistic and antioxidant effect of probiotic Weissella cibaria JW15. Food Sci Biotechnol 28(3):851–855. https://doi.org/10.1007/s10068-018-0519-6
Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L (2005) Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B Analyt Technol Biomed Life Sci 827(1):76–82. https://doi.org/10.1016/j.jchromb.2005.06.035
Ighodaro OM, Akinloye OA (2019) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Guerin P, El Mouatassim S, Menezo Y (2001) Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum reprod update 7(2):175–189. https://doi.org/10.1093/humupd/7.2.175
Ho YS, **ong Y, Ma W, Spector A, Ho DS (2004) Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279(31):32804–32812. https://doi.org/10.1074/jbc.M404800200
Achuthan AA, Duary RK, Madathil A, Panwar H, Kumar H, Batish VK, Grover S (2012) Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol Biol Rep 39(8):7887–7897. https://doi.org/10.1007/s11033-012-1633-9
Wu T, Zhang Y, Lv Y, Li P, Yi D, Wang L, Zhao D, Chen H, Gong J, Hou Y (2018) Beneficial impact and molecular mechanism of Bacillus coagulans on piglets’ intestine. Int J Mol Sci 19 (7). https://doi.org/10.3390/ijms19072084
Suzuki T, Yamamoto M (2017) Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem 292(41):16817–16824. https://doi.org/10.1074/jbc.R117.800169
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36(10):1208–1213. https://doi.org/10.1016/j.freeradbiomed.2004.02.075
Li C, Cheng L, Wu H, He P, Zhang Y, Yang Y, Chen J, Chen M (2018) Activation of the KEAP1NRF2ARE signaling pathway reduces oxidative stress in Hep2 cells. Mol Med Rep 18(3):2541–2550. https://doi.org/10.3892/mmr.2018.9288
Rahman MS, Hee Choi Y, Seok Choi Y, Alam MB, Han Lee S, Cheol Yoo J (2018) A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem 239:502–510. https://doi.org/10.1016/j.foodchem.2017.06.106
Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29(2):493–502. https://doi.org/10.1128/MCB.01080-08
Gao D, Gao Z, Zhu G (2013) Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct 4(6):982–989. https://doi.org/10.1039/c3fo30316k
Wang LX, Liu K, Gao DW, Hao JK (2013) Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol 19(20):3150–3156. https://doi.org/10.3748/wjg.v19.i20.3150
Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L, Rosser R, Wolkowitz OM, Mellon SH (2017) Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76:197–205. https://doi.org/10.1016/j.psyneuen.2016.11.031
Biswas SK (2016) Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev 2016:5698931. https://doi.org/10.1155/2016/5698931
Xu LQ, **e YL, Gui SH, Zhang X, Mo ZZ, Sun CY, Li CL, Luo DD, Zhang ZB, Su ZR, **e JH (2016) Polydatin attenuates d-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct 7(11):4545–4555. https://doi.org/10.1039/c6fo01057a
Yang G, Shen K, Yu R, Wu Q, Yan Q, Chen W, Ding L, Kumar V, Wen C, Peng M (2020) Probiotic (Bacillus cereus) enhanced growth of Pengze crucian carp concurrent with modulating the antioxidant defense response and exerting beneficial impacts on inflammatory response via Nrf2 activation. Aquaculture 529. https://doi.org/10.1016/j.aquaculture.2020.735691
Acknowledgements
This research was supported by Liaoning Province Natural Science Foundation of China (2020-MZLH-39), scientific research fund project of Liaoning Provincial Department of Education (J2019017).
Author information
Authors and Affiliations
Contributions
**aoxi Gao and Yuhong Zhang performed the experiments and wrote the manuscript. Yunpeng Xu, **nmiao Wang checked the logicality and language of this manuscript. Fang Qian, Yanfeng Tuo and Guangqing Mu conceived and designed the study. Fang Qian was responsible for overall study coordination of this manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animals.
Conflict of Interest
The authors state that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, X., Zhang, Y., Mu, G. et al. Protecting Effect of Bacillus coagulans T242 on HT-29 Cells Against AAPH-Induced Oxidative Damage. Probiotics & Antimicro. Prot. 14, 741–750 (2022). https://doi.org/10.1007/s12602-022-09917-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09917-5