Abstract
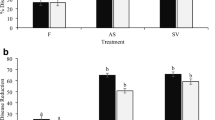
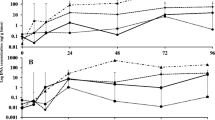
The efficacy of alkaline extracts from Acanthophora spicifera, Gracilaria ornata, Codium taylorii, Caulerpa serrulata, and Sargassum vulgare was assessed both in vitro and in planta under greenhouse conditions. Seaweeds were collected from the coast of Trinidad, WI. Alkaline extracts were tested for their efficacy in promoting plant growth and performance in tomato (Solanum lycopersicum, Hybrid-61) and sweet pepper (Capsicum annuum, Amrit) and for their efficacy in controlling infections caused by Alternaria solani and Xanthomonas campestris pv. vesicatoria. Germination assays indicated that seed bio-priming with seaweed extracts lead to a significant increase in seed germination percentage (up to 21.7%), germination index (up to 21.86%) and seedling vigour index (up to 105.8%) compared to hydro-primed seeds. Mean germination time was also significantly reduced (up to 40.6%) in seaweed bio-primed seeds. Chlorophyll and carotenoid contents significantly increased (up to 18.3 and 42.5%, respectively) in seedlings that were bio-primed with seaweed extracts. Greenhouse trials showed that plants foliar sprayed with 0.5% v/v seaweed extract had significantly fewer incidences of bacterial spot (22.9–49.1% and 24.6–49.2% for sweet pepper and tomato, respectively) and early blight (32.8–48.3% and 31.9–47.5% for sweet pepper and tomato, respectively). Furthermore, seaweed extract treatments caused a significant increase in the fruit yield of tomato (118.2-181.8%) and sweet pepper (47.1–70.6%). In vitro antimicrobial assay revealed that all seaweed extracts had no direct antimicrobial effect against a panel of phytopathogens. However, upon foliar treatments with the extracts, the DNA levels of A. solani and X. campestris pv. vesicatoria were significantly lower compared to the controls. Seaweed extract-treated tomato and sweet pepper plants had significantly higher activities of chitinase, peroxidase, polyphenol oxidase, phenylalanine ammonia-lyase, glucanase and higher levels of total phenols. Concurrently, the upregulation of marker genes involved in the salicylic acid, jasmonic acid, and ethylene-mediated defence pathways was also observed in seaweed extract-treated plants. The above results point towards the elicitor-like activity of seaweed extracts on plants’ metabolic processes. This study, therefore, highlights the beneficial effects of extracts from Caribbean seaweeds and thus supports their potential use in plant systems as a sustainable crop input.






Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Ali, N., Farrell, A., Ramsubhag, A., & Jayaraj, J. (2016). The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. Journal of Applied Phycology, 28(2), 1353–1362. https://doi.org/10.1007/s10811-015-0608-3
Ali, O., Ramsubhag, A., Daniram Benn, S., Jr, & Jayaraj, J. (2022). Transcriptomic changes induced by applications of a commercial extract of Ascophyllum nodosum on tomato plants. Scientific Reports, 12(1), 1–13. https://doi.org/10.1038/s41598-022-11263-z
Ali, O., Ramsubhag, A., & Jayaraj, J. (2018). Ascophyllum nodosum (Linnaeus) Le Jolis seaweed extract improves seed germination in tomato and sweet pepper under NaCl-induced salt stress. Tropical Agriculture, 95(July 2019), 141–148.
Ali, O., Ramsubhag, A., & Jayaraj, J. (2019). Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS One, 14(5), 140–155. https://doi.org/10.1371/journal.pone.0216710
Ali, O., Ramsubhag, A., & Jayaraj, J. (2021a). Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants, 10(3):1–27. https://doi.org/10.3390/plants10030531
Ali, O., Ramsubhag, A., & Jayaraj, J. (2021b). Phytoelicitor activity of Sargassum vulgare and Acanthophora spicifera extracts and their prospects for use in vegetable crops for sustainable crop production. Journal of Applied Phycology, 33(1), 639–651. https://doi.org/10.1007/s10811-020-02309-8
Aranda, P. S., Lajoie, D. M., & Jorcyk, C. L. (2012). Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis, 33(2). https://doi.org/10.1002/elps.201100335
Borad, V., & Sriram, S. (2008). Pathogenesis-related proteins for the plant protection. Asian Journal of Experimental Sciences, 22(3), 189–196.
Bazoobandi, M., Rahimi, H., & Karimi-Shahri, M. R. (2020). Saffron crop protection. Saffron, 169–185. https://doi.org/10.1016/B978-0-12-818638-1.00010-1
Bekele, D., & Birhan, M. (2021). The impact of secondary macro nutrients on crop production. International Journal of Research Studies in Agricultural Sciences, 7, https://doi.org/10.20431/2454-6224.0705005
Berlin, A., Källström, H. N., Lindgren, A., & Olson, Å. (2018). Scientific evidence for sustainable plant disease protection strategies for the main arable crops in Sweden. A systematic map protocol. Environmental Evidence, 7. https://doi.org/10.1186/s13750-018-0141-3
Brent, K. J., Hollomon, D. W. (2007). Fungicide Resistance in Crop Pathogens: How Can it be Managed? FRAC Monograph 1. 2nd Ed. Brussels, CropLife International, Brussels: 55.
Choudhary, A., Kumar, A., & Kaur, N. (2020). ROS and oxidative burst: Roots in plant development. Plant Diversity, 42(1). https://doi.org/10.1016/j.pld.2019.10.002
Chaerani, R., & Voorrips, R. E. (2006). Tomato early blight (Alternaria solani):the pathogen, genetics, and breeding for resistance. Journal of General Plant Pathology, 72, 335–347.
Crouch, I. J., Beckett, R. P., & van Staden, J. (1990). Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. Journal of Applied Phycology, 2(3), 269–272. https://doi.org/10.1007/BF02179784
Da Silva, M. B. P., Silva, V. N., & Vieira, L. C. (2021). Biopriming of sweet pepper and tomato seeds with ascophyllum nodosum. Revista Facultad Nacional de Agronomia Medellin, 74(1), 55–76. https://doi.org/10.15446/rfnam.v74n1.88240
Dookie, M., Ali, O., Ramsubhag, A., & Jayaraman, J. (2021). Flowering gene regulation in tomato plants treated with brown seaweed extracts. Scientia Horticulturae, 276. https://doi.org/10.1016/j.scienta.2020.109715
du Jardin, P. (2015). Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae, 196:3–14. https://doi.org/10.1016/j.scienta.2015.09.021
Duncan, E. J., & Lee Lum, L. M. (2006). A checklist of the marine macroalgae of the Republic of Trinidad and Tobago. Caribbean Marine Studies, 7(3), 1–96.
El Boukhari, M. E. M., Barakate, M., Bouhia, Y., & Lyamlouli, K. (2020). Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants, 9, 111–131.
El-Din, S. M. (2015). Utilization of seaweed extracts as bio-fertilizers to stimulate the growth of wheat seedlings. Egyptian Journal of Experimental Biology (Botany) 11(1), 31–39
El-Sheekh M, Ismail M, Hamouda M (2016) Influence of Some Brown Seaweed Extracts on Germination and Cytological Responses of Trigonella foenum-graecum L. BioTechnol: An Indian Journal Research, 12(6), 104.
El Modafar, C., Elgadda, M., El Boutachfaiti, R., Abouraicha, E., Zehhar, N., Petit, E., Alaoui-Talibi, E., Courtois, Z., & Courtois, J. (2012). Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Horticulturae, 138(5), 55–63. https://doi.org/10.1016/j.scienta.2012.02.011
Eugenia, M., Carvalho, A., Roberto, P., Dionisia, A., Novembre, L. C., Carvalho, M. E. A., Castro, P. R. C., Novembre, A. D. C., & Chamma, H. M. C. P. (2013). Seaweed extract improves the Vigor and provides the Rapid Emergence of Dry Bean Seeds. Journal of Agriculture and Environmental Sciences, 13, 8.
Fan, D., Hodges, D. M., Critchley, A. T., & Prithiviraj, B. (2013). A commercial extract of Brown Macroalga (Ascophyllum nodosum) affects yield and the Nutritional Quality of Spinach in Vitro. Communications in Soil Science and Plant Analysis, 44(12), 1873–1884. https://doi.org/10.1080/00103624.2013.790404
Geiger, F., Bengtsson, J., Berendse, F., Weisser, W. W., Emmerson, M., Morales, M. B., Ceryngier, P., Liira, J., Tscharntke, T., Winqvist, C., Eggers, S., Bommarco, R., Pärt, T., Bretagnolle, V., Plantegenest, M., Clement, L. W., Dennis, C., Palmer, C., Oñate, J. J., & Inchausti, P. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential on european farmland. Basic and Applied Ecology, 11(2). https://doi.org/10.1016/j.baae.2009.12.001
Hammerschmidt, R., Nuckles, E. M., & Kuć, J. (1982). Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiological Plant Pathology. https://doi.org/10.1016/0048-4059(82)90025-X
Hamouda, M. M., Saad-Allah, K. M., & Gad, D. (2022). Potential of seaweed extract on growth, physiological, cytological and biochemical parameters of wheat (Triticum aestivum L.) seedlings. Journal of Soil Science and Plant Nutrition. https://doi.org/10.1007/s42729-022-00774-3
Hernández-Herrera, R. M., Santacruz-Ruvalcaba, F., Ruiz-López, M. A., Norrie, J., & Hernández-Carmona, G. (2014). Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). Journal of Applied Phycology, 26(1), 619–628. https://doi.org/10.1007/s10811-013-0078-4
Hidangmayum, A., & Sharma, R. (2017). Effect of different concentration of commercial seaweed liquid extract of Ascophylum nodosum on germination of onion (Allium cepa L.). International Journal of Science and Research (IJSR), 6(7), 1488–1491. https://doi.org/10.21275/art20175686
Jannin, L., Arkoun, M., Etienne, P., Laîné, P., Goux, D., Garnica, M., Fuentes, M., Francisco, S. S., Baigorri, R., Cruz, F., Houdusse, F., Garcia-Mina, J. M., Yvin, J. C., Ourry, A., & Metabolisms, S. (2013). Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. Seaweed extract: microarray analysis and physiological characterization of N. Journal of Plant Growth Regulation, 32(1), 31–52. https://doi.org/10.1007/s00344-012-9273-9
Jayaraj, J., Wan, A., Rahman, M., & Punja, Z. K. (2008). Seaweed extract reduces foliar fungal diseases on carrot. Crop Protection, 27(10), 1360–1366. https://doi.org/10.1016/j.cropro.2008.05.005
Jayaraj, J., Norrie, J., & Punja, Z. K. (2011). Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. Journal of Applied Phycology, 23(3), 353–361. https://doi.org/10.1007/s10811-010-9547-1
Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., Critchley, A. T., Craigie, J. S., Norrie, J., & Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 45(4), 112–134. https://doi.org/10.1007/s00344-009-9103-x
Khopade, M. (2015). A preliminary study on the effects of ozone on induction of resistance in Cicer arietinum and Trigonella foenum against acute ozone exposure. IOSR Journal of Biotechnology and Biochemistry, 1(5), 6–14.
Klarzynski, O., Descamps, V., Plesse, B., Yvin, J. C., Kloareg, B., & Fritig, B. (2003). Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Molecular Plant-Microbe Interactions, 16(2), 115–122. https://doi.org/10.1094/MPMI.2003.16.2.115
Kunkel, B. N., & Brooks, D. M. (2002). Cross talk between signaling pathways in pathogen defense. Current Opinion Plant Biology, 5, 325–331. https://doi.org/10.1016/S1369-5266(02)00275-3
Lai, Y., Xu, B., He, L., Lin, M., Cao, L., Mou, S., Wu, Y., & He, S. (2011). Differential gene expression in pepper (Capsicum annuum) exposed to UV-B. Indian Journal of Experimental Biology, 49(6), 429–437.
Laing, W., & Christeller, J. (2004). Extraction of proteins from plant tissues. Current Protocols in Protein Science / Editorial Board John E Coligan … et Al ]. https://doi.org/10.1002/0471140864.ps0407s38
Mahuku, G. S. (2004). A simple extraction method suitable for PCR-based analysis of plant, fungal, and bacterial DNA. Plant Molecular Biology Reporter, 22(1), 71–81. https://doi.org/10.1007/BF02773351
Makhaye, G., Aremu, A. O., Gerrano, A. S., Tesfay, S., Du Plooy, C. P., & Amoo, S. O. (2021). Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants, 10(7). https://doi.org/10.3390/plants10071327
Malathi, S., & Mohan, S. (2013). Induction of defence-related enzymes in onion by combined application of fungal and bacterial biocontrol agents against Fusarium oxysporum f. sp. cepae. Archives of Phytopathology and Plant Protection, 46(2), 243–252. https://doi.org/10.1080/03235408.2012.737724
Maoka, T. (2020). Carotenoids as natural functional pigments. Journal of Natural Medicines, 74(1). https://doi.org/10.1007/s11418-019-01364-x
Michalak, I., Dmytryk, A., Schroeder, G., & Chojnacka, K. (2017). The application of homogenate and filtrate from baltic seaweeds in seedling growth tests. Applied Sciences (Switzerland), 7(3). https://doi.org/10.3390/app7030230
Mishra, M., Mahajan, N., Tamhane, V. A., Kulkarni, M. J., Baldwin, I. T., Gupta, V. S., & Giri, A. P. (2012). Stress inducible proteinase inhibitor diversity in Capsicum annuum BMC Plant Biology, 12(6), 54–66. https://doi.org/10.1186/1471-2229-12-217
Morgan, J. B., & Connolly, E. L. (2013). Plant – Soil interactions: nutrient uptake. Nature Education Knowledge, 4(8), 2
Nazeem, P. A., Achuthan, C. R., Babu, T. D., Parab, G. V., Girija, D., Keshavachandran, R., & Samiyappan, R. (2008). Expression of pathogenesis related proteins in black pepper (Piper nigrum L.) in relation to Phytophthora foot rot disease. Journal of Tropical Agriculture, 46(1–2), 33–39.
Oñate-Sánchez, L., & Singh, K. B. (2002). Identification of arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiology, 128(4), 1313–1322. https://doi.org/10.1104/pp.010862
Pan, S. Q., Ye, X. S., & Kuć, J. (1991). Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiological and Molecular Plant Pathology, 39(1), 167–177. https://doi.org/10.1016/0885-5765(91)90029-H
Rajendran, R., Jagmohan, S., Jayaraj, P., Ali, O., Ramsubhag, A., & Jayaraj, J. (2022). Effects of Ascophyllum nodosum extract on sweet pepper plants as an organic biostimulant in grow box home garden conditions. Journal of Applied Phycology, 34(1). https://doi.org/10.1007/s10811-021-02611-z
Ramirez-Estrada, K., Vidal-Limon, H., Hidalgo, D., Moyano, E., Golenioswki, M., Cusidó, R. M., & Palazon, J. (2016). Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. https://doi.org/10.3390/molecules21020182
Ramkissoon, A., Ramsubhag, A., & Jayaraj, J. (2017). Phytoelicitor activity of three caribbean seaweed species on suppression of pathogenic infections in tomato plants. Journal of Applied Phycology, 29(6), 3235–3244. https://doi.org/10.1007/s10811-017-1160-0
Reddy, S. A., Bagyaraj, D. J., & Kale, R. D. (2012). Management of tomato bacterial spot caused by Xanthomonas campestris using vermicompost. Journal of Biopesticides, 5(1), 10–13
Rouphael, Y., & Colla, G. (2020). Editorial: Biostimulants in agriculture. Frontiers in Plant Science, 11. https://doi.org/10.3389/fpls.2020.00040
Saha, P., & Das, S. (2012). Assessment of yield loss due to early blight (Alternaria solani) in tomato. Indian Journal Plant Protection, 40, 195–198
Sangha, J. S., Kelloway, S., Critchley, A. T., & Prithiviraj, B. (2014). Seaweeds (Macroalgae) and their extracts as contributors of plant productivity and quality. The current status of our understanding. Advances in Botanical Research, 21(4), 132–133. https://doi.org/10.1016/B978-0-12-408062-1.00007-X
Shukla, P. S., Mantin, E. G., Adil, M., Bajpai, S., Critchley, A. T., & Prithiviraj, B. (2019). Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Frontiers in Plant Science, 10, 401–415. https://doi.org/10.3389/fpls.2019.00655
Silva-Beltrán, N. P., Ruiz-Cruz, S., Cira-Chávez, L. A., Estrada-Alvarado, M. I., Ornelas-Paz, J. D. J., López-Mata, M. A., Del-Toro-Sánchez, C. L., Ayala-Zavala, J. F., & Márquez-Ríos, E. (2015). Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. International Journal of Analytical Chemistry, 2(1), 11–21. https://doi.org/10.1155/2015/284071
Stadnik, M. J., & de Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Tropical Plant Pathology, 39(2). https://doi.org/10.1590/S1982-56762014000200001
Vandecasteele, M., Landschoot, S., Carrette, J., Verwaeren, J., Höfte, M., Audenaert, K., & Haesaert, G. (2018). Species prevalence and disease progression studies demonstrate a seasonal shift in the Alternaria population composition on potato. Plant Pathology, 67(2), 327–336. https://doi.org/10.1111/ppa.12734
Van Dijk, L. J. A., Ehrlén, J., & Tack, A. J. M. (2021). Direct and insect-mediated effects of pathogens on plant growth and fitness. Journal of Ecology, 109. https://doi.org/10.1111/1365-2745.13689
Van Oosten, M. J., Pepe, O., De Pascale, S., Silletti, S., & Maggio, A. (2017). The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chemical and Biological Technologies in Agriculture, 4, 5. https://doi.org/10.1186/s40538-017-0089-5.
Yakhin O. I., Lubyanov, A. A., Yakhin, I. A., Brown, P. H. (2017). Biostimulants in plant science: A global perspective. Front Plant Sci 7:23–27. https://doi.org/10.3389/fpls.2016.02049.
Zhang, Y. L., Li, D. W., Gong, Z. H., Wang, J. E., Yin, Y. X., & Ji, J. J. (2013). Genetic determinants of the defense response of resistant and susceptible pepper (Capsicum annuum) cultivars infected with Phytophthora capsici (oomycetes; Pythiaceae). Genetics and Molecular Research, 12(3), 3605–3621. https://doi.org/10.4238/2013.September.13.5
Zulfiqar, F. (2021). Effect of seed priming on horticultural crops. Scientia Horticulturae, 286. https://doi.org/10.1016/j.scienta.2021.110197
Acknowledgements
This work was supported in part by the UWI-CRP Grant, UWI-RDI Fund and SARGOOD-FED-INTERREG V-Caraïbes Research Programme. The authors wish to thank the greenhouse staff at the Department of Life Sciences, The University of the West Indies for hel** with the experimental setup. Thanks to Mr Stephen Daniram Benn Jr. Ramnarine for critical reading of the manuscript.
Funding
This work was funded in part by the UWI-CRP Grant, UWI-RDI Fund and SARGOOD-FED-INTERREG V-Caraïbes Research Programme.
Author information
Authors and Affiliations
Contributions
Omar Ali: experimentation, methodology, data collection, curation and data analysis and writing the original manuscript.
Adesh Ramsubhag: analysis, methodology, supervision, review and editing of the manuscript.
Jayaraj Jayaraman: conceptualization, funding acquisition, project administration, methodology, review and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, O., Ramsubhag, A. & Jayaraman, J. Application of extracts from Caribbean seaweeds improves plant growth and yields and increases disease resistance in tomato and sweet pepper plants. Phytoparasitica 51, 727–745 (2023). https://doi.org/10.1007/s12600-022-01035-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01035-w