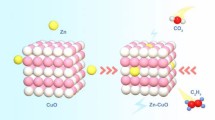
Abstract
Efficient bifunction electrocatalyst is extremely interesting for electrochemical overall water splitting (OWS). Herein, a new RuO2-Ru/MoO2@CC (RRM/CC) bifunctional electrocatalyst was prepared via a solid phase reaction strategy. To obtain a suitable precursor for SPR, MoS2 nanosheets and RuO2 nanoparticles (NPs) were sequentially loaded onto carbon cloth conductive substrate. Subsequently, the prepared RuO2/MoS2/CC precursor was sealed in a furnace and annealed in Ar to trigger the redox SPR. After SPR, active RuO2-Ru/MoO2 units containing metal–metal oxide interfaces were formed on CC substrate uniformly. The optimized RRM/CC sample annealed at 400 °C exhibited a overpotential of 13 mV for hydrogen evolution reaction (HER) and 231 mV for oxygen evolution reaction (OER) at 10 mA·cm−2 under alkaline condition, respectively, which can be deduced to the modulated electronic structure and unique hierarchical structure. In addition, a low cell voltage of 1.48 V for OWS was required at 10 mA·cm−2 under alkaline condition. Meanwhile, RRM/CC exhibited excellent pH-independent durability.
Graphical abstract

摘要
高效双功能电催化剂对于电化学全水分解(OWS)非常有趣。在此,通过固相反应(SPR)策略制备了一种新型RuO2-Ru/MoO2@CC(RRM/CC)双功能电催化剂。将MoS2纳米片和RuO2纳米粒子依次负载到碳布(CC)导电基底上得到SPR的前驱体。随后,将制备的RuO2/MoS2/CC前驱体Ar中退火以触发两者间固相反应。得到在CC基底上均匀形成含有金属-金属氧化物界面的活性RuO2-Ru/MoO2单元。在400 oC退火的优化RRM/CC电催化剂在碱性条件下在10 mA cm−2下的析氢反应(HER)和析氧反应(OER)的过电势分别为13 mV和231 mV,这归因于优异的电子传达和独特的异质结构。此外,在碱性条件下,10 mA cm−2 下的OWS电压仅为1.48 V。与此同时,RRM/CC在全pH条件下均表现出优异的稳定性。






Similar content being viewed by others
References
Mallapaty S. How China could be carbon neutral by mid-century. Nature. 2020;586(7830):482. https://doi.org/10.1038/d41586-020-02927-9.
Qiu Y, Liu SQ, Wei C, Fan JX, Yao H, Dai LX, Wang GM, Li H, Su BL, Guo XH. Synergistic effect between platinum single atoms and oxygen vacancy in MoO2 boosting pH-universal hydrogen evolution reaction at large current density. Chem Eng J. 2022;427:131309. https://doi.org/10.1016/j.cej.2021.131309.
Li YR, Li MX, Li SN, Liu YJ, Chen J, Wang Y. A review of energy and environment electrocatalysis based on high-index faceted nanocrystals. Rare Met. 2021;40(12):3406. https://doi.org/10.1007/s12598-021-01747-8.
Xu QC, Zhang JH, Zhang HX, Zhang LY, Chen L, Hu YJ, Jiang H, Li CZ. Atomic heterointerface engineering overcomes the activity limitation of electrocatalysts and promises highly-efficient alkaline water splitting. Energy Environ Sci. 2021;14:5228. https://doi.org/10.1039/D1EE02105B.
Wang LF, Yang RX, Fu JZ, Cao YY, Ding RF, Xu XH. Vertically aligned W(Mo)S2/N-W(Mo)C-based light-assisted electrocatalysis for hydrogen evolution in acidic solutions. Rare Met. 2023;42(5):1535. https://doi.org/10.1007/s12598-022-02250-4.
Xu JY, Liu TF, Li JJ, Li B, Liu YF, Zhang BS, **ong DH, Amorim I, Li W, Liu LF. Boosting the hydrogen evolution performance of ruthenium clusters through synergistic coupling with cobalt phosphide. Energy Environ Sci. 2018;11:1819. https://doi.org/10.1039/C7EE03603E.
Lv HL, Zhang XD, Cai JL, **e X, Fan YX, Liu LY, Ding J, Cai Q, Liu YS. Construction of RuSe2/MoOx hybrid and used as bi-functional electrocatalyst for overall water splitting. Mater Chem Phys. 2022;277:125461. https://doi.org/10.1016/j.matchemphys.2021.125461.
Liu SQ, Dai LX, Qu YT, Fan JX, Li X, Zhang QH, Guo XH. Crystalline/amorphous hetero-phase Ru nanoclusters for efficient electrocatalytic oxygen reduction and hydrogen evolution. Mater Chem Front. 2021;5(17):6648. https://doi.org/10.1039/d1qm00812a.
Deng X, Zheng XD, Gong ZW, Tan WY, Pei XD. Research progress on single metal atom catalysts for hydrogen production by PEM water electrolysis with lower costs. Chin J Rare Met. 2023;47(01):43. https://doi.org/10.13373/j.cnki.cjrm.XY22060014.
An CH, Kang W, Deng QB, Hu N. Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range. Rare Met. 2022;41(2):378. https://doi.org/10.1007/s12598-021-01791-4.
Deng LM, Hu F, Ma MY, Huang SC, **ong YX, Chen HY, Li LL, Peng SJ. Electronic modulation caused by interfacial Ni-O-M (M = Ru, Ir, Pd) bonding for accelerating hydrogen rvolution kinetics. Angew Chem Int Ed. 2021;60(41):22276. https://doi.org/10.1002/anie.202110374.
Zhang LJ, Jang H, Wang Y, Li ZJ, Zhang W, Kim MG, Yang DJ, Liu SG, Liu X, Cho J. Exploring the dominant role of atomic- and nano-ruthenium as active sites for hydrogen evolution reaction in both acidic and alkaline media. Adv Sci. 2021;8(15):2004516. https://doi.org/10.1002/advs.202004516.
Gao QS, Zhang WB, Shi ZP, Yang LC, Tang Y. Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv Mater. 2019;31(2):1802880. https://doi.org/10.1002/adma.201802880.
Yang Y, Su JW, Jiang P, Chen JT, Hu L, Chen QW. MOFs-derived N-doped carbon-encapsulated metal/alloy electrocatalysts to tune the electronic structure and reactivity of carbon active sites. Chin J Chem. 2021;39(9):2626. https://doi.org/10.1002/cjoc.202100207.
**e X, Fan YX, Tian WY, Zhang M, Cai JL, Zhang XA, Ding J, Liu YS, Lu SY. Construction of Ru/WO3 with hetero-interface structure for efficient hydrogen evolution reaction. J Energy Chem. 2023;83:150. https://doi.org/10.1016/j.jechem.2023.04.026.
Hua W, Sun HH, Xu F, Wang JG. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020;39(4):335. https://doi.org/10.1007/s12598-020-01384-7.
Wang N, Ning SL, Yu XL, Chen D, Li ZL, Xu JC, Meng H, Zhao DK, Li LG, Liu QM, Lu BZ, Chen SW. Graphene composites with Ru-RuO2 heterostructures: highly efficient mott–schottky-type electrocatalysts for pH-universal water splitting and flexible zinc–air batteries. Appl Catal B. 2022;302:120838. https://doi.org/10.1016/j.apcatb.2021.120838.
Zhang CC, Wei S, Sun LX, Ouyang YF, Xu F, Chu HL, Pan HG, Chen LX, **ao XZ. Enhanced hydrogen evolution performance by 3D ordered macroporous Ru-CoP@NC electrocatalysts. Rare Met. 2023. https://doi.org/10.1007/s12598-023-02494-8.
Fan YX, Zhang XD, Zhang YJ, **e X, Ding J, Cai JL, Li BJ, Lv HL, Liu LY, Zhu MM, Zheng XC, Cai Q, Liu YS, Lu SY. Decoration of Ru/RuO2 hybrid nanoparticles on MoO2 plane as bifunctional electrocatalyst for overall water splitting. J Colloid Interface Sci. 2021;604:508. https://doi.org/10.1016/j.jcis.2021.07.038.
Zhang MT, Chen JX, Li H, Cai PW, Li Y, Wen ZH. Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting within 0.73 V in an asymmetric-electrolyte electrolyzer. Nano Energy. 2019;61:576. https://doi.org/10.1016/j.nanoen.2019.04.050.
Guo ML, Wu ZY, Zhang MM, Huang ZJ, Zhang KX, Wang BR, Tu JC. Coupling interface constructions of FeOOH/NiCo2S4 by microwave-assisted method for efficient oxygen evolution reaction. Rare Met. 2023;42(6):1847. https://doi.org/10.1007/s12598-022-02239-z.
Dong H, Zan ZH, Zhou X. Theoretical investigation on structures, stabilities, and hydrolysis reactions of small RuO2 nanoclusters. Chin J Chem. 2014;32(6):527. https://doi.org/10.1002/cjoc.201300912.
Zhang FF, Gu XD, Zheng SJ, Yuan HF, Li JP, Wang XG. Highly catalytic flexible RuO2 on carbon fiber cloth network for boosting chlorine evolution reaction. Electrochim Acta. 2019;307:385. https://doi.org/10.1016/j.electacta.2019.03.187.
Li YG, Wang Y, Lu JM, Yang B, San XY, Wu ZS. 2D intrinsically defective RuO2/graphene heterostructures as all-pH efficient oxygen evolving electrocatalysts with unprecedented activity. Nano Energy. 2020;78:105185. https://doi.org/10.1016/j.nanoen.2020.105185.
Cai JL, Ding J, Wei DH, **e X, Li BJ, Lu SY, Zhang JM, Liu YS, Cai Q, Zang SQ. Coupling of Ru and O-vacancy on 2D Mo-based electrocatalyst via a solid-phase interface reaction strategy for hydrogen evolution reaction. Adv Energy Mater. 2021;11(26):2100141. https://doi.org/10.1002/aenm.202100141.
Lin JH, Wang PC, Wang HH, Li C, Si XQ, Qi JL, Cao J, Zhong ZX, Fei WD, Feng JC. Defect-rich heterogeneous MoS2/NiS2 nanosheets electrocatalysts for efficient overall water splitting. Adv Sci. 2019;6(14):1900246. https://doi.org/10.1002/advs.201900246.
Cai JL, Yang JY, ** triggered efficient electrochemical hydrogen evolution of cross-linked porous Ru-MoO2 via solid-phase reaction strategy. Energy Environ Mater. 2022;6(1):e12424. https://doi.org/10.1002/eem2.12424.
Wu JB, Su JW, Wu T, Huang L, Li Q, Luo YX, ** HR, Zhou J, Zhai TY, Wang DS, Gogotsi Y, Li YD. Scalable synthesis of 2D Mo2C and thickness-dependent hydrogen evolution on its basal plane and edges. Adv Mater. 2023. https://doi.org/10.1002/adma.202209954.
Bian LZ, Gao W, Sun JM, Han MM, Li FL, Gao ZF, Shu L, Han N, Yang ZX, Song A, Qu YQ, Ho JC. Phosphorus-doped MoS2 nanosheets supported on carbon cloths as efficient hydrogen-generation electrocatalysts. ChemCatChem. 2018;10(7):1571. https://doi.org/10.1002/cctc.201701680.
Zhan GM, Quan FJ, Yao YC, Zhao SX, Liu XF, Gu HY, Huang Y, Liu X, Jia FL, Zhang LZ. Single-layer MoS2 with adjacent Mo sites for efficient electrocatalytic nitrogen fixation via spin-delocalized electrons effect. Appl Catal B. 2023;323:122186. https://doi.org/10.1016/j.apcatb.2022.122186.
Li XH, Han SH, Qiao ZL, Zeng XF, Cao DP, Chen JF. Ru monolayer island doped MoS2 catalysts for efficient hydrogen evolution reaction. Chem Eng J. 2023;453:139803. https://doi.org/10.1016/j.cej.2022.139803.
Huang C, Ruan QD, Song H, Luo Y, Bai HZ, Gao B, Chu PK. Vertical kinetically oriented MoS2–Mo2N heterostructures on carbon cloth: a highly efficient hydrogen evolution electrocatalyst. Sustain Energ Fuels. 2020;4(5):2201. https://doi.org/10.1039/d0se00144a.
Zhu LL, Wang Z, Li CD, Li H, Huang YN, Li H, Wu ZQ, Lin S, Li N, Zhu XB, Sun YP. Highly stable 1T-MoS2 by magneto-hydrothermal synthesis with Ru modification for efficient hydrogen evolution reaction. J Mater Chem A. 2022;10(39):21013. https://doi.org/10.1039/D2TA05954A.
Guo XW, Song E, Zhao W, Xu SM, Zhao WL, Lei YJ, Fang YQ, Liu JJ, Huang FQ. Charge self-regulation in 1T-MoS2 structure with rich S vacancies for enhanced hydrogen evolution activity. Nat Commun. 2022;13(1):5954. https://doi.org/10.1038/s41467-022-33636-8.
Sun X, Zhao Y, Chang K, Peng B, Gu QQ, Yang B, Yu BY, Xu J, Liu FD, Zhang Y, Pan CS, Lou Y. 1T-phase MoS2 edge-anchored Pt1−S3 active site boosting selective hydrogenation of biomass-derived maleic anhydride. Rare Met. 2023;42(8):2658. https://doi.org/10.1007/s12598-023-02299-9.
Qin R, Wang PY, Li ZL, Zhu JX, Cao F, Xu HW, Ma QL, Zhang JY, Yu J, Mu SC. Ru-incorporated nickel diselenide nanosheet arrays with accelerated adsorption kinetics toward overall water splitting. Small. 2022;18(6):2105305. https://doi.org/10.1002/smll.202105305.
Cheng Y, Zhou X, Pan QM, Zhang LF, Cao YF, Qian T. Bimetallic active site nuclear-shell heterostructure enables efficient dual-functional electrocatalysis in alkaline media. Rare Met. 2023;42(9):3024. https://doi.org/10.1007/s12598-023-02300-5.
Han Y, Manjunath C, Yu G, Wang CY, Chao YF, Simonov AN, Wallace GG. Binder-free electrodes derived from interlayer-expanded MoS2 nanosheets on carbon cloth with a 3D porous structure for lithium storage. ChemElectroChem. 2019;6(8):2338. https://doi.org/10.1002/celc.201900270.
Chen YY, Zhang Y, Ma YL, Tang T, Dai ZH, Hu JS, Wan LJ. Facile synthesis of Mo2C nanocrystals embedded in nanoporous carbon network for efficient hydrogen evolution. Chin J Chem. 2017;35(6):911. https://doi.org/10.1002/cjoc.201600790.
Sun X, Chen HJ, Yin YM, Curnan MT, Han JW, Chen Y, Ma ZF. Progress of exsolved metal nanoparticles on oxides as high performance (electro)catalysts for the conversion of small molecules. Small. 2021;17(10):2005383. https://doi.org/10.1002/smll.202005383.
Wu T, Sun MZ, Huang BL. Non-noble metal-based bifunctional electrocatalysts for hydrogen production. Rare Met. 2022;41(7):2169. https://doi.org/10.1007/s12598-021-01914-x.
Acknowledgements
This work was financially supported by Henan Provincial University Science and Technology Innovation Team (No. 24IRTSTHN008). The authors thank the Center for Modern Analysis and Gene Sequencing of Zhengzhou University in support of the characterization.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, JL., Fan, JY., Zhang, XD. et al. Construction of RuO2-Ru/MoO2@carbon cloth bifunctional electrocatalyst for efficient overall water splitting. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02772-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02772-z