Abstract
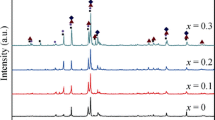
LaMgNi4−x Co x (x = 0–0.8) electrode alloys used for MH/Ni batteries were prepared by induction melting. The structures and electrochemical hydrogen storage properties of the alloys were investigated in detail. X-ray diffraction (XRD) and scanning electron microscopy (SEM) analysis show that LaMgNi4 phase and LaNi5 phase are obtained. The lattice parameters of the two phases increase first and then decrease with Co content increasing. The electrochemical properties of the alloy electrodes were measured by means of simulated battery tests. Results show that the addition of Co does not change the discharge voltage plateau of the alloy electrodes. However, the maximum discharge capacity increases from 319.9 mAh·g−1 (x = 0) to 347.5 mAh·g−1 (x = 0.4) and then decreases to 331.7 mAh·g−1 (x = 0.8). The effects of Co content on electrochemical kinetics of the alloy electrodes were also performed. The high rate dischargeability (HRD) first increases and then decreases with Co content increasing and reaches the maximum value (95.0 %) when x = 0.4. Test results of the electrochemical impedance spectra (EIS), potentiodynamic polarization curves and constant potential step measurements of the alloy electrodes all demonstrate that when Co content is 0.4 at%, the alloy exhibits the best comprehensive electrochemical properties.








Similar content being viewed by others
References
Zhao DL, Zhang YH. Research progress in Mg-based hydrogen storage alloys. Rare Met. 2014;33(5):499.
Jiang L, Li GX, Xu LQ, Jiang WQ, Lan ZQ, Guo J. Effect of substituting Mn for Ni on the hydrogen storage and electrochemical properties of ReNi2.6−x Mn x Co0.9 alloys. Int J Hydrogen Energy. 2010;35(1):204.
Kohno T, Yoshida H, Kawashima F, Inaba T, Sakai I, Yamamoto M, Kanda M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J Alloys Compd. 2000;311(2):L5.
Aono K, Orimo S, Fujii H. Structural and hydriding properties of MgYNi4: a new intermetallic compound with C15b-type Laves phase structure. J Alloys Compd. 2000;309(1–2):L1.
Kadir K, Noréus D, Yamashita I. Structural determination of AMgNi4 (where A = Ca, La, Ce, Pr, Nd and Y) in the AuBe5 type structure. J Alloys Compd. 2002;345(1–2):140.
Wang ZM, Ni CY, Zhou HY, Yao QR. Structural characterization of REMgNi4 type compounds. Mater Charact. 2008;59(4):422.
Teresiak A, Uhlemann M, Thomas J, Eckert J, Gebert A. Influence of Co and Pd on the formation of nanostructured LaMg2Ni and its hydrogen reactivity. J Alloys Compd. 2014;582:647.
Tian X, Yun GH, Wang HY, Shang T, Yao ZQ, Wei W, Liang XX. Preparation and electrochemical properties of La–Mg–Ni-based La0.75Mg0.25Ni3.3Co0.5 multiphase hydrogen storage alloy as negative material of Ni/MH battery. Int J Hydrogen Energy. 2014;39(16):8474.
Liu YX, Xu LQ, Jiang WQ, Li GX, Wei WL, Guo J. Effect of substituting Al for Co on the hydrogen-storage performance of La0.7Mg0.3Ni2.6Al x Co0.5−x (x = 0–0.3) alloys. Int J Hydrogen Energy. 2009;34(7):2986.
Tan JB, Zeng XQ, Zou JX, Wu XM, Ding WJ. Preparation of LaMgNi4−x Co x alloys and hydrogen storage properties. Trans Nonferr Met Soc China. 2013;23(8):2307.
Zhang SL, Zhou HY, Wang ZM, Zou RP, Xu HR. Preparation and electrode properties of NdMgNi4−x Co x hydrogen storage alloys. J Alloys Compd. 2005;398(1–2):269.
Zhang SL, Zhao YT, Chen G, Cheng XN, Sun HQ. Preparation and electrode behavior of NdMgNi3.6Co0.4 hydrogen storage alloy. J Mater Process Technol. 2008;198(1–3):270.
Zhang FL, Luo YC, Wang DH, Yan RX, Kang L, Chen JH. Structure and electrochemical properties of La2−x Mg x Ni7.0 (x = 0.3–0.6) hydrogen storage alloys. J Alloys Compd. 2007;439(1–2):181.
Liu YF, Cao YH, Huang L, Gao MX, Pan HG. Rare earth–Mg–Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries. J Alloys Compd. 2011;509(3):675.
Yang T, Zhai TT, Yuan ZM, Bu WG, Xu S, Zhang YH. Hydrogen storage properties of LaMgNi3.6M0.4 (M = Ni Co, Mn, Cu, Al) alloys. J Alloys Compd. 2014;617:29.
Pu ZG, Zhu YF, Zhu JY, Yuan JG, Zhang JG, Chen W, Fang JJ, Li LQ. Kinetics and electrochemical characteristics of Mg2NiH4−x MmNi3.8Co0.75Mn0.4Al0.2 (x = 5, 10, 20, 40) composites for Ni-MH battery. Int J Hydrogen Energy. 2014;39(8):3887.
Chu HL, Zhang Y, Qiu SJ, Qi YN, Sun LX, Xu F, Wang Q, Dong C. Electrochemical performances of cobalt-free La0.7Mg0.3Ni3.5−x (MnAl2) x (x = 0–0.20) hydrogen storage alloy electrodes. J Alloys Compd. 2008;457(1–2):90.
Kuriyama N, Sakai T, Miyamura H, Uehara I, Ishikawa H, Iwasaki T. Electrochemical impedance and deterioration behavior of metal hydride electrodes. J Alloys Compd. 1993;202(1–2):183.
Pan HG, Ma JX, Wang CS, Chen SA, Wang XH, Chen CP, Wang QD. Studies on the electrochemical properties of MlNi4.3−x Co x Al0.7 hydride alloy electrodes. J Alloys Compd. 1999;293–295:648.
Lin J, Cheng Y, Liang F, Sun LS, Yin DM, Wu YM, Wang LM. High temperature performance of La0.6Ce0.4Ni3.45Co0.75Mn0.7Al0.1 hydrogen storage alloy for nickel/metal hydride batteries. Int J Hydrogen Energy. 2014;39(25):13231.
Liu YF, Pan HG, Yue YJ, Wu XF, Chen N, Lei YQ. Cycling durability and degradation behavior of La–Mg–Ni–Co-type metal hydride electrodes. J Alloys Compd. 2005;395(1–2):291.
Witham C, Hightower A, Fultz B, Ratnakumar BV, Bowman RC Jr. Electrochemical properties of LaNi5−x Ge x alloys in Ni-MH batteries. J Electrochem Soc. 1997;144(11):3758.
Dong XP, Lü FX, Yang LY, Zhang YH, Wang XL. Influence of spark plasma sintering temperature on electrochemical performance of La0.80Mg0.20Ni3.75 alloy. Mater Chem Phys. 2008;112(2):596.
Ratnakumar BV, Witham C, Bowman RC Jr, Hightower A, Fultz B. Electrochemical studies on LaNi5−x Sn x metal hydride alloys. J Electrochem Soc. 1996;143(8):2578.
Liu YF, Pan HG, Gao MX, Wang QD. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J Mater Chem. 2011;21(13):4743.
Zheng G, Popov BN, White RE. Electrochemical determination of the diffusion-coefficient of hydrogen through an LaNi4.25Al0.75 electrode in alkaline aqueous-solution. J Electrochem Soc. 1995;142(8):2695.
Dong ZW, Ma LQ, Shen XD, Wang LM, Wu YM, Wang LD. Cooperative effect of Co and Al on the microstructure and electrochemical properties of AB3-type hydrogen storage electrode alloys for advanced MH/Ni secondary battery. Int J Hydrogen Energy. 2011;36(1):893.
Zhang YH, Yang T, Shang HW, Zhao C, Xu C, Zhao DL. The electrochemical hydrogen storage characteristics of as-spun nanocrystalline and amorphous Mg20Ni10−x M x (M = Cu Co, Mn; x = 0–4) alloys. Rare Met. 2014;33(6):663.
Zhang YH, Ren HP, Cai Y, Yang T, Zhang GF, Zhao DL. Structures and electrochemical hydrogen storage performance of Si added A2B7-type alloy electrodes. Trans Nonferr Met Soc China. 2014;24(2):406.
Acknowledgments
This work was financially supported by the National Natural Science Foundations of China (Nos. 51161015, 51371094 and 51471054).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Zhai, TT., Yuan, ZM. et al. Structure and electrochemical properties of LaMgNi4−x Co x (x = 0–0.8) hydrogen storage electrode alloys. Rare Met. 37, 249–256 (2018). https://doi.org/10.1007/s12598-016-0734-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0734-3