Abstract
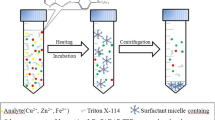
Cloud point extraction (CPE) with Tergitol TMN-6 was applied for the extraction of trace amounts of palladium (Pd(II)), platinum (Pt(IV)), and gold (Au(III)) in the soil of industrial sewage. Ammonium pyrolysine dithiocarbamate (APDC) was adopted as the chelating agent prior to CPE and then was detected by atomic absorption spectrometry (AAS). Different parameters such as the concentration of surfactants, chelating agent and salt, sample pH, equilibration temperature and time, centrifugation time and rates, and the effect of foreign ions were studied. Under optimum conditions, the low limits of detections are 1.4, 2.8 and 1.2 ng·ml−1 and the enrichment factors are 21, 12, and 24 for Pd(II), Pt(IV), and Au(III), respectively. The relative standard deviations vary from 0.6% to 1.0% (n=11). All correlation coefficients of the calibration curves are >0.9960. The proposed method was successfully applied for the determination of Pd(II), Pt(IV), and Au(III) in the real soil of industrial sewage samples.
Similar content being viewed by others
References
Farago M.E., Kavanag P., Blanks R., Kelly J., Kazantzis G., Tornton I., Simpson P.R., Cook J.M., Parry S., and Hall G.E.M., Platinum-catalyzed enzyme electrodes immobilized on gold using self-assembled layers, J. Anal. Chem., 1998, 70(11): 2396.
Balcerzak M., Analytical methods for the determination of platinum in biological and environmental materials, J. Anal. Chem., 2011, 41(3): 214.
Akira Morikawa, Kohei Okumura, Masaru Ishii, Koichi Kikuta, Akihiko Suda, and Hirofumi Shinjo, Characterization of termetallic Pt-Ir-Au catalysts for NO decomposition, Rare Metals., 2011, 30(1): 53.
**ong C.H., Zhu S., Yao C.P., and Chen Q., Adsorption behavior of Pd(II) from aqueous solutions by D201 resin, J. Rare Metals, 2011, 30(1): 470.
Medved J., Bujdos M., Matus P., and Kubova J., Determination of trace amounts of gold in acid-attacked environmental samples by atomic absorption spectrometry with electrothermal atomization after preconcentration, Anal. Bioanal. Chem., 2004, 379(1): 60.
Underwood E.J., Trace Elements in Human and Animal Nutrition, Academic Press, New York, 1977: 92.
Tsalev D.L., and Zaprianov Z.K., Atomic Absorption Spectrometry in Occupational and Environmental Health Practice, CRC Press, Boca Raton, Florida, 1985: 104.
Krishna Priya B., Subrahmanayam P., Suvardhan K., Suresh Kumar K., Rekha D., Venkata Rao A., Rao G.C., and Chiranjeevi P., Cloud point extraction of palladium in water samples and alloy mixtures using new synthesized reagent with flame atomic absorption spectrometry (FAAS), J. Hazard. Mater., 2007, 144(2): 152.
Shaibal I.M., Khanom F., Rahman M.A., and Alam A.M.S., Separation and determination of metals in mixed solvent system on anion exchanger using atomic absorption spectrophotometer, J. Anal. Chem., 2005, 6(5): 35.
Kumar K.S., Suvardhan K., Krishnaiah L., and Chiranjeevi P., Novel reactions for the simple and sensitive spectrophotometric determination of traces of selenium in environmental samples, Helv. Chim. Acta, 2005, 88(2): 343.
Li H.Z., and Zhai Y.B., Solid-phase extraction of trace Au(III) with SDG and determination by the catalytic spectrophotometric method, Rare Metals, 2008, 27(6): 560.
Zhou Q.X., Zhao N., and **e G.H., Determination of lead in environmental waters with dispersive liquid-liquid microextraction prior to atomic fluorescence spectrometry, J. Hazard. Mater., 2011, 189(1): 48.
Luconi M.O., Olsina R.A., Fern’andez L.P., and Silva M.F., Determination of lead in human saliva by combined cloud point extraction-capillary zone electrophoresis with indirect UV detection, J. Hazard. Mater., 2006, 128(2): 240.
Singh P., Mittal S., and Sharma R.K., Solid phase extraction and estimation of cadmium using glycine immobilized cellulose chelating resin, Indian J. Chem. Technol., 2007, 14(2): 204.
Carson B.L., Ellis III H.V., and McCann J.L., Toxicology and Biological Monitoring of Metals in Humans, Lewis Publishers, Chelsea, UK, 1986: 51.
Lindqvist O., Environmental impact of mercury and other heavy metals, J. Power Sources., 1995, 57(5): 3.
Stalikas C.D., Micelle-mediated extraction as a tool for separation and preconcentration in metal analysis, Trends Anal. Chem., 2002, 21(5): 343.
Ghaedi M., Shokrollahi A., Ahmadi F., Rajabi H.R., and Soylak M., Cloud point extraction for the determination of copper, nickel and cobalt ions in environ mental samples by flame atomic absorption spectrometry, J. Hazard. Mater., 2008, 150(2): 533.
Martins I.M., Rodrigues S.N., Barreiro M.F., and Rodrigues A.E., Polylactide-based thyme oil microcapsules production: evaluation of surfactants, Ind. Eng. Chem. Res., 2011, 50(2):898.
Soylak M., Elc L.I., and Dogan M., Determination of trace amounts of cobalt in natural water samples as 4-(2-thiazolylazo) recorcinol complex after adsorptive preconcentration, Anal. Lett., 1997, 30(4): 623.
Ince M., Kaplan O., and Yaman M., Solid-phase extraction and preconcentration of copper in mineral waters with 4-(2-pyridyl-azo) resorcinol-loaded amberlite XAD-7 and flame atomic absorption spectrometry, Water Environ. Res., 2008, 80(11): 2104.
Yang G., Fen W., Lei C., **ao W., and Sun H., Study on solid phase extraction and graphite furnace atomic absorption spectrometry for the determination of nickel, silver, cobalt, copper, cadmium and lead with MCI GEL CHP 20Y as sorbent, J. Hazard. Mater., 2009, 162(1): 44.
Jan S., Waqar F., and Khan S.A., Extraction of traces of chromium, copper and lead from water using fluorinated beta-diketone immobilized on styrenedivinylbenzene as chelating resin, J. Chem. Soc. Pakistan., 2007, 29(2): 476.
Shabani A.M.H., Dadfarnia S., Shahbaazi Z., and Jafari A.A., Extraction spectrophotometric determination of nickel at microgram level in water and wastewater using 2-[(2-mercaptophenylimino) methyl] phenol, Bull. Chem. Soc. Ethiopia., 2008, 22(3): 323.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lian, Y., Zhen, W., Tai, Z. et al. Cloud point extraction and flame atomic absorption spectrometry analysis of palladium, platinum, and gold ions from industrial polluted soil. Rare Metals 31, 512–516 (2012). https://doi.org/10.1007/s12598-012-0549-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-012-0549-9