Abstract
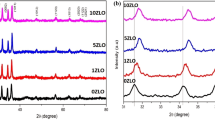
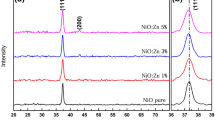
In this research, we investigated the structural, electrical, and sensing characteristics of undoped and doped TiO2 thin films prepared through the spray pyrolysis technique, using titanium tetrachloride as the precursor material. The thin films were deposited on quartz and silicon substrates, maintaining a substrate temperature of 350 ºC, and subjected to annealing at 550 ºC for 120 min. X-ray diffraction (XRD) analysis revealed the polycrystalline nature of the films, and the crystallite size was calculated using Scherrer's formula. Nickel-doped titanium oxide thin films were also fabricated, varying the do** concentrations (Ni/Ti = 0%, 1%, 2%, 3%, 4%, and 5%), deposited on quartz substrates via the spray pyrolysis technique. The impact of do** on the structural and optical properties was explored, with X-ray diffraction indicating polycrystalline thin films exhibiting an anatase phase structure. Optical analysis demonstrated a reduction in the optical band gap with increasing do** concentration in TiO2 thin films.


















Similar content being viewed by others
Data availability
On request, the corresponding author will provide you with the data sets generated during this investigation.
References
A.M. Al-Zuhery, S.M. Al-Jawad, A.K. Al-Mousoi, The effect of PbS thickness on the performance of CdS/PbS solar cell prepared by CSP. Optik (Stuttg). 130, 666–672 (2017). https://doi.org/10.1016/j.ijleo.2016.10.120
D. Bouras, M. Fellah, A. Mecif, R. Barillé, A. Obrosov, M. Rasheed, High photocatalytic capacity of porous ceramic-based powder doped with MgO. J. Korean Ceram. Soc. (2022). https://doi.org/10.1007/s43207-022-00254-5
D. Bouras, M. Rasheed, R. Barille, M.N. Aldaraji, Efficiency of adding DD3+(Li/Mg) composite to plants and their fibers during the process of filtering solutions of toxic organic dyes. Opt. Mater. 131, 112725 (2022). https://doi.org/10.1016/j.optmat.2022.112725
A. Pottier, C. Chanéac, E. Tronc, L. Mazerolles, J.-P. Jolivet, Synthesis of brookite TiO2 nanoparticles by thermolysis of TiCl4 in strongly acidic aqueous media. J. Mater. Chem. 11(4), 1116–1121 (2001). https://doi.org/10.1039/b100435m
L. Usgodaarachchi, C. Thambiliyagodage, R. Wijesekera, S. Vigneswaran, M. Kandanapitiye, Fabrication of TiO2 spheres and a visible light active α-Fe2O3/TiO2-Rutile/TiO2-Anatase heterogeneous photocatalyst from natural ilmenite. ACS Omega 7(31), 27617–27637 (2022). https://doi.org/10.1021/acsomega.2c03262
V. Vrakatseli, E. Farsari, D. Mataras, Wetting properties of transparent anatase/rutile mixed phase glancing angle magnetron sputtered nano-TiO2 films. Micromachines 11(6), 616 (2020). https://doi.org/10.3390/mi11060616
Gayan, H. Hakkoum, E. Comini, Recent advancements in TiO2 nanostructures: sustainable synthesis and gas sensing. Nanomaterials 13(8), 1424–1424 (2023). https://doi.org/10.3390/nano13081424
M.L. ArunaKumari, L. Gomathi Devi, G. Maia, T.-W. Chen, N. Al-Zaqri, M. Ajmal Ali, Mechanochemical synthesis of ternary heterojunctions TiO2(A)/TiO2(R)/ZnO and TiO2(A)/TiO2(R)/SnO2 for effective charge separation in semiconductor photocatalysis: A comparative study. Environ Res 203, 111841–111841 (2022). https://doi.org/10.1016/j.envres.2021.111841
Selma, M.R. Mohammad, N.J. Imran, Effect of electrolyte solution on structural and optical properties of tio2 grown by anodization technique for photoelectrocatalytic application. Surface Rev Lett 25(04), 1850078–1850078 (2018). https://doi.org/10.1142/s0218625x18500786
O.N. Hussein, Selma, N.J. Imran, Structural, morphological, optical, and antibacterial properties of Mn and (Ag, Mn) co-doped copper sulfide nanostructures. Opt. Quantum. Electron. 55(6) (2023). https://doi.org/10.1007/s11082-023-04769-x
M. Rasheed, R. Barillé, Room temperature deposition of ZnO and Al:ZnO ultrathin films on glass and PET substrates by DC sputtering technique. Opt. Quantum. Electron. 49(5) (2017). https://doi.org/10.1007/s11082-017-1030-7
Y.-C. Liang, W.-C. Zhao, Morphology-dependent photocatalytic and gas-sensing functions of three-dimensional TiO2–ZnO nanoarchitectures. CrystEngComm (2020). https://doi.org/10.1039/d0ce01036g
K.H. Aboud, S.M.H. AL-Jawad, N.J. Imran, Synthesis and characterization of flakes-like and flowers-like Ni: CdS nano films via hydrothermal technique for photocatalytic activity, J. Mater. Sci. Mater. Electron. 34 (2023). https://doi.org/10.1007/s10854-023-10330-z
E. Kadri et al., Ac conductivity and dielectric behavior of a−Si:H/c−Si1−yGey/p−Si thin films synthesized by molecular beam epitaxial method. J. Alloy. Compd. 705, 708–713 (2017). https://doi.org/10.1016/j.jallcom.2017.02.117
K.H. Aboud, S.M.H. AL-Jawad, N.J. Imran, Preparation and Characterization of Hierarchical CdS Nanoflowers for Efficient Photocatalytic Degradation. J. Nanostructures. 12, 316–329 (2022)
N. Ben Azaza et al., “3-(p-nitrophenyl)Coumarin derivatives: Synthesis, linear and nonlinear optical properties. Opt Mater 96, 109328 (2019). https://doi.org/10.1016/j.optmat.2019.109328
H.N.C. Dharma et al., A review of titanium dioxide (TiO2)-based photocatalyst for oilfield-produced water treatment. Membranes 12(3), 345 (2022). https://doi.org/10.3390/membranes12030345
N. Farooq et al., Recent trends of titania (TiO2) based materials: a review on synthetic approaches and potential applications. J. King Saud. Univ. Science/Maǧallaẗ ǧāmiʹaẗ al-malik Saʹūd. al-ʹUlūm, pp 103210–103210 (2024). https://doi.org/10.1016/j.jksus.2024.103210
J. Reszczyńska et al., Lanthanide co-doped TiO2: the effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 307, 333–345 (2014). https://doi.org/10.1016/j.apsusc.2014.03.199
W. Zhang et al., Interfacial lattice‐strain‐driven generation of oxygen vacancies in an Aerobic‐Annealed TiO2(B) electrode. Adv Mater 31(52) (2019). https://doi.org/10.1002/adma.201906156
B. Choudhury, A. Choudhury, Tailoring luminescence properties of TiO2 nanoparticles by Mn do**. J. Lumin. 136, 339–346 (2013). https://doi.org/10.1016/j.jlumin.2012.12.011
X. Tian et al., Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater Sci 3(4), 390–403 (2021). https://doi.org/10.1016/j.nanoms.2021.05.011
M.V. Castro et al., Optimisation of surface treatments of TiO2: Nb transparent conductive coatings by a post-hot-wire annealing in a reducing H2 atmosphere. Thin Solid Films 550, 404–412 (2014). https://doi.org/10.1016/j.tsf.2013.11.044
S. Cao, N. Sui, P. Zhang, T. Zhou, J. Tu, T. Zhang, TiO2 nanostructures with different crystal phases for sensitive acetone gas sensors. J. Colloid Interface Sci. 607, 357–366 (2022). https://doi.org/10.1016/j.jcis.2021.08.215
S.M. Patil et al., NH3 gas sensing performance of ternary TiO2/SnO2/WO3 hybrid nanostructures prepared by ultrasonic-assisted sol–gel method. J. Mater. Sci. Mater. Electron. 29(14), 11830–11839 (2018). https://doi.org/10.1007/s10854-018-9283-x
Y. **ong et al., Gas sensing capabilities of TiO2 porous nanoceramics prepared through premature sintering. J. Adv. Ceram. 4(2), 152–157 (2015). https://doi.org/10.1007/s40145-015-0148-y
B. Varun Kumar, S. Darshan, B.W. Shivaraj, M. Krishna, G. SatheeshBabu, C. Manjunatha, Modeling and simulation of TiO2/Se sensor for detection of CO gas using comsol multiphysics. ECS Trans 107(1), 5867–5877 (2022). https://doi.org/10.1149/10701.5867ecst
Z. Lou, F. Li, J. Deng, L. Wang, T. Zhang, Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): a novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces. 5(23), 12310–12316 (2013). https://doi.org/10.1021/am402532v
T. Gao, B.P. Jelle, Thermal conductivity of TiO2 nanotubes. J Phys Chem C 117(3), 1401–1408 (2013). https://doi.org/10.1021/jp3108655
N.R. D'Amico, G. Cantele, D. Ninno, First principles calculations of the band offset at SrTiO3−TiO2 interfaces. Appl. Phys. Lett. 101(14) (2012). https://doi.org/10.1063/1.4757281
M.H. Seo, M. Yuasa, T. Kida, J.S. Huh, N. Yamazoe, K. Shimanoe, Detection of organic gases using TiO2 nanotube-based gas sensors. Procedia. Chem. 1(1), 192–195 (2009). https://doi.org/10.1016/j.proche.2009.07.048
D. Mardare et al., Low temperature TiO2 based gas sensors for CO2. Ceram. Int. 42(6), 7353–7359 (2016). https://doi.org/10.1016/j.ceramint.2016.01.137
S. Chakraborty, An investigation on the long-term stability of TiO2 nanofluid. Mater Today: Proc 11, 714–718 (2019). https://doi.org/10.1016/j.matpr.2019.03.032
D. Mondal, A.M. Nair, S. Mukherji, Volatile organic compound sensing in breath using conducting polymer coated chemi-resistive filter paper sensors. Med. Biol. Eng. Compu. 61(8), 2001–2011 (2023). https://doi.org/10.1007/s11517-023-02861-8
S. Saini, A. Lodhi, A. Dwivedi, A. Khandelwal, S.P. Tiwari, Resistive switching behavior of TiO2/(PVP:MoS2) Nanocomposite bilayer hybrid RRAM. Commun. Compu. Inf. Sci. pp 478–485 (2022). https://doi.org/10.1007/978-3-031-21514-8_39.
H.K. Judran, N.A. Yousif, S.M.H. AL-Jawad, Preparation and characterization of CdS prepared by hydrothermal method. J. Sol-Gel. Sci. Technol. (2020). https://doi.org/10.1007/s10971-020-05430-9
S.M.H. Al-Jawad, M.M. Ismail, Characterization of Mn, Cu, and (Mn, Cu) co-doped ZnS nanoparticles. J. Opt. Technol. 84(7), 495 (2017). https://doi.org/10.1364/jot.84.000495
Selma, N.J. Imran, K.H. Aboud, Synthesis and characterization of Mn:CdS nanoflower thin films prepared by hydrothermal method for photocatalytic activity. J Sol-gel Science Technol 100(3), 423–439 (2021). https://doi.org/10.1007/s10971-021-05656-1
Selma, M.H. AL-Jawad, N.J. Imran, M.R. Mohammad, Effect of electrolyte solution and deposition methods on TiO2/CdS core–shell nanotube arrays for photoelectrocatalytic application. European. Phys. J. Appl. Phys. 92(2): 20102–20102 (2020). https://doi.org/10.1051/epjap/2020200127
M. Enneffatia, M. Rasheed, B. Louatia, K. Guidaraa, S. Shihab, R. Barillé, “Investigation of structural, morphology, optical properties and electrical transport conduction of Li0.25Na0.75CdVO4 compound. J Phys: Conf Ser 1795(1), 012050 (2021). https://doi.org/10.1088/1742-6596/1795/1/012050
M. Rasheed, O.Y. Mohammed, S. Shihab, A. Al-Adili, Explicit numerical model of solar cells to determine current and voltage. J. Phys: Conf. Ser. 1795(1), 012043 (2021). https://doi.org/10.1088/1742-6596/1795/1/012043
M. Rasheed, SuhaShihab, O. Alabdali, H.H. Hassan, Parameters extraction of a single-diode model of photovoltaic cell using false position iterative method. J Phys: Conf Ser 1879(3), 032113 (2021). https://doi.org/10.1088/1742-6596/1879/3/032113
M. Rasheed, S. Shihab, O.Y. Mohammed, A. Al-Adili, Parameters Estimation of Photovoltaic Model Using Nonlinear Algorithms. J. Phys: Conf. Ser. 1795(1), 012058 (2021). https://doi.org/10.1088/1742-6596/1795/1/012058
M.A. Sarhan, S. Shihab, B.E. Kashem, M. Rasheed, New exact operational shifted pell matrices and their application in astrophysics. J. Phys: Conf. Ser. 1879(2), 022122 (2021). https://doi.org/10.1088/1742-6596/1879/2/022122
The Ky Vo, Mo-modified TiO2 mesoporous microspheres prepared by spray pyrolysis for adsorption-photocatalytic water remediation. J. Sol-Gel. Sci. Technol. 103(3), 853–864 (2022). https://doi.org/10.1007/s10971-022-05902-0
E. Kadri, M. Krichen, R. Mohammed, A. Zouari, K. Khirouni, Electrical transport mechanisms in amorphous silicon/crystalline silicon germanium heterojunction solar cell: impact of passivation layer in conversion efficiency. Opt Quantum Electron 48(12) (2016). https://doi.org/10.1007/s11082-016-0812-7
S.D. Sharma et al., Sol–gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst. Appl. Catal. A 314(1), 40–46 (2006). https://doi.org/10.1016/j.apcata.2006.07.029
M. Crişan et al., Sol–gel S-doped TiO2 materials for environmental protection. J. Non-Cryst. Solids 354(2–9), 705–711 (2008). https://doi.org/10.1016/j.jnoncrysol.2007.07.083
V. Mansfeldova et al., Work function of TiO2 (Anatase, Rutile, and Brookite) single crystals: effects of the environment. J. Phys. Chem. C 125(3), 1902–1912 (2021). https://doi.org/10.1021/acs.jpcc.0c10519
S. Nasiri et al., Modified Scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends 3, 100015 (2023). https://doi.org/10.1016/j.nwnano.2023.100015
K. Nakata, A. Fujishima, TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol., C 13(3), 169–189 (2012). https://doi.org/10.1016/j.jphotochemrev.2012.06.001
M. Sellam, M. Rasheed, S. Azizi, T. Saidani, Improving photocatalytic performance: Creation and assessment of nanostructured SnO2 thin films, pure and with nickel do**, using spray pyrolysis. Ceram. Int. (2024). https://doi.org/10.1016/j.ceramint.2024.03.094
K. Fukushima, I. Yamada, Surface smoothness and crystalline structure of ICB deposited TiO2 films. Appl. Surf. Sci. 43(1–4), 32–36 (1989). https://doi.org/10.1016/0169-4332(89)90186-4
M. Mansoor, An interaction effect of perceived government response on COVID-19 and government agency’s use of ICT in building trust among citizens of Pakistan. Trans Gov: People Process Policy ahead-of-print, no. ahead-of-print, (2021). https://doi.org/10.1108/tg-01-2021-0002
C. Liu et al. High performance, biocompatible dielectric thin‐film optical filters integrated with flexible substrates and microscale optoelectronic devices. Adv Opt Mater 6(15) (2018). https://doi.org/10.1002/adom.201800146
Y. Wu, S. **ng, J. Fu, Examining the use of TiO2 to enhance the NH3 sensitivity of polypyrrole films. J. Appl. Polym. Sci. 118(6), 3351–3356 (2010). https://doi.org/10.1002/app.32382
P. Desai et al. Synthesis and dielectric studies of Ni doped MgO nanostructure. Inorg. Nano-metal Chem. pp 1–13 (2023) https://doi.org/10.1080/24701556.2023.2260373
L.A. Patil, D.N. Suryawanshi, I.G. Pathan, D.M. Patil, Nickel doped spray pyrolyzed nanostructured TiO2 thin films for LPG gas sensing. Sens. Actuators, B Chem. 176, 514–521 (2013). https://doi.org/10.1016/j.snb.2012.08.030
Acknowledgements
The authors are thankful for the computing resources of the Universite d’Angers, Laboratory Moltech Anjou and in Angers, France and University of Technology- Iraq of Iraq.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
S. A., M. R. and Z. A.; methodology, S. A., M. R. and Z. A.; planned and conducted the tests, S. A. and M. R.; data analysis and interpretation, and M. R. and S. A.; prepared the manuscript. All authors have read and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethical approval
Not Applicable.
Conflict of interest or computing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AL-Jawad, S.M.H., RASHEED, M. & Abbas, Z.Y. Effect of do** on the structural, optical and electrical properties of TiO2 thin films for gas sensor. J Opt (2024). https://doi.org/10.1007/s12596-024-01913-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12596-024-01913-y