Abstract
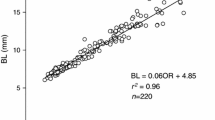
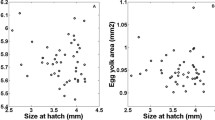
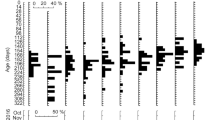
We conducted a rearing experiment on Pacific bluefin tuna (PBT) larvae, which originated from one female broodstock, and determined the growth history of the larvae to clarify when the growth difference occurs. We investigated the otolith microstructure of the PBT larvae to examine the individual growth history and to predict the age and body size at the onset of individual growth difference. Since total length (TL) of fish can be back-calculated from otolith radius, we back-calculated TLs of 100 fish of 19 days post hatch (dph) divided into three size groups (small, intermediate, large). Growth difference was recognized from 3 dph (mouth opening), and the difference became larger thereafter. Growth of large-size fish was assumed to be promoted by the feeding conditions of PBT larvae.





Similar content being viewed by others
References
Tucker JW (1998) Characteristics of marine fish. In: Tucker JW (ed) Marine fish culture. Kluwer Academic, Norwell, pp 17–48
Matsui I (1949) Genetical studies of the Carp. II. On the morphological characteristics of Tobi-koi (Superior growing carp’s group). J Shimonoseki Coll Fish 1:27–32 (in Japanese with English abstract)
Matsui I (1950) Studies on the growth of the pond-cultured animals. V. On the growth of Tobi-koi (superior growing carp’s group). Ichthyol Res 1:166–174 (in Japanese with English abstract)
Nakamura N, Kasahara S (1955) A study on the phenomenon of the Tobi-Koi or shoot carp-I. On the earliest stage at which the shoot carp appears. Nippon Suisan Gakkaishi 21:73–76 (in Japanese with English abstract)
Danzmann RG, Ferguson MM (1995) Heterogeneity in the body size of Ontario cultured rainbow trout with different mitochondrial DNA haplotypes. Aquaculture 137:231–244
Sekino M, Saitoh K, Yamada T, Kumagai A, Hara M, Yamashita Y (2003) Microsatellite-based pedigree tracing in a Japanese flounder Paralichthys olivaceus hatchery strain: implications for hatchery management related to stock enhancement program. Aquaculture 221:255–263
Nakamura N, Kasahara S (1956) A study on the phenomenon of the Tobi-Koi or shoot carp-II. On the effect of particle size and quantity of the food. Nippon Suisan Gakkaishi 21:1022–1024 (in Japanese with English abstract)
Petursdottir TE (2002) Influence of feeding frequency on growth and size dispersion in arctic charr Salvelinus alpinus (L.). Aquacult Res 33:543–546
Umino T, Otsu M, Takaba M, Nakagawa H (1993) Some characteristics of runty fish appearing in seed production of red sea bream. Nippon Suisan Gakkaishi 59:925–928
Adams C, Huntingford F, Turnbull J, Arnott S, Bell A (2000) Size heterogeneity can reduce aggression and promote growth in Atlantic salmon parr. Aquacult Int 8:543–549
Sakakura Y, Tsukamoto K (1996) Onset and development of cannibalistic behaviour in early life stages of yellowtail. J Fish Biol 48:16–29
Moran D (2007) Size heterogeneity, growth potential and aggression in juvenile yellowtail kingfish (Seriola lalandi Valenciennes). Aquacult Res 38:1254–1264
Georgiadis MP, Hedrick RP, Johnson WO, Gardner IA (2000) Mortality and recovery of runt white sturgeon (Acipenser transmontanus) in a commercial farm in California, USA. Prev Vet Med 43:269–281
Sawada Y, Okada T, Miyashita S, Murata O, Kumai H (2005) Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) life cycle. Aquacult Res 36:413–421
Sabate FS, Sakakura Y, Tanaka Y, Kumon K, Nikaido H, Eba T, Nishi A, Shiozawa S, Hagiwara A, Masuma S (2010) Onset and development of cannibalistic and schooling behavior in the early life stages of Pacific bluefin tuna Thunnus orientalis. Aquaculture 301:16–21
Masuma S, Takebe T, Sakakura Y (2011) A review of the broodstock management and larviculture of the Pacific northern bluefin tuna in Japan. Aquaculture 315:2–8
Miyashita S (2006) Surfacing and bottoming death in seedling production. Nippon Suisan Gakkaishi 72:947–948 (in Japanese)
Tanaka Y, Kumon K, Nishi A, Eba T, Nikaido H, Shiozawa S (2009) Status of the sinking of hatchery-reared larval Pacific bluefin tuna on the bottom of the mass culture tank with different aeration design. Aquacult Sci 57:587–593
Sawada Y, Miyashita S, Aoyama M, Kurata M, Mukai Y, Okada T, Murata O, Kumai H (2000) Rotifer-size selectivity and optimal feeding density of bluefin tuna, Thunnus thynnus, larvae. Aquacult Sci 48:169–177
Miyashita S (2002) Studies on the seedlings production of the Pacific bluefin tuna, Thunnus thynnus orientalis. Bull Fish Lab Kinki Univ 8:1–171 (in Japanese)
Seoka M, Kurata M, Kumai H (2007) Effect of docosahexaenoic acid enrichment in Artemia on growth of Pacific bluefin tuna Thunnus orientalis larvae. Aquaculture 270:193–199
Sabate FS, Sakakura Y, Takebe T, Nikaido H, Matsumoto N, Shiozawa S, Hagiwara A, Masuma S (2009) Preliminary observations on the development of aggressive behavior in Pacific bluefin tuna Thunnus orientalis. Aquacult Sci 57:329–335
Itoh T, Shiina Y, Tsuji S, Endo F, Tezuka N (2000) Otolith daily increment formation in laboratory reared larval and juvenile bluefin tuna Thunnus thynnus. Fish Sci 66:834–839
Campana SE (1990) How reliable are growth back-calculations based on otolith? Can J Fish Aquat Sci 47:2219–2227
Campana SE, Jones CM (1992) Analysis of microstructure data. Can Spec Publ Fish Aquat Sci 117:73–100
Miyashita S, Sawada Y, Okada T, Miyashita S, Murata O, Kumai H (2001) Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae). Fish Bull 99:601–616
Tanaka Y, Satoh K, Iwahashi M, Yamada H (2006) Growth-dependent recruitment of Pacific bluefin tuna Thunnus orientalis in the northwestern Pacific Ocean. Mar Ecol Prog Ser 319:225–235
Nakamura N (1955) Comparison of the Carp and the Crucian carp from pisci-cultural point of view. Nippon Suisan Gakkaishi 21:77–81 (in Japanese with English abstract)
Shoji J, Maehara T, Tanaka M (1999) Short-term occurrence and rapid growth of Spanish mackerel larvae in the central waters of the Seto Inland Sea, Japan. Fish Sci 65:68–72
Masuda R, Shoji J, Aoyama M, Tanaka M (2002) Chub mackerel larvae fed fish larvae can swim faster than those fed rotifers and Artemia nauplii. Fish Sci 68:320–324
Dan S, Koiso M (2008) Effect of microalgal addition on stability of n-3HUFA contents in enriched rotifer Brachionus plicatilis in large tank for seed production. Aquacult Sci 56:603–604 (in Japanese with English abstract)
Yamamoto T, Teruya K, Hara T, Hokazono H, Kai I, Hashimoto H, Furuita H, Matsunari H, Mushiake K (2009) Nutritional evaluation of rotifers in rearing tanks without water exchange during the post-hatch period in seed production of amberjack Serola dumerili. Fish Sci 75:697–705
Tomoda T, Koiso M, Kuwada H, Chen JN, Takeuchi T (2004) Dietary value of marine rotifer Brachionus plicatilis in different population growth stages for larval red sea bream Pagrus major. Nippon Suisan Gakkaishi 70:573–582 (in Japanese with English abstract)
Tomoda T, Koiso M, Kuwada H, Chen JN, Takeuchi T (2005) Dietary value of marine rotifer Brachionus plicatilis at different population growth stages for larval Japanese flounder Paralichthys olivaceus. Nippon Suisan Gakkaishi 71:555–562 (in Japanese with English abstract)
Miyashita S, Tanaka Y, Sawada Y, Murata O, Hattori N, Takii K, Mukai Y, Kumai H (2000) Embryonic development and effects of water temperature on hatching of the bluefin tuna, Thunnus thynnus. Aquaculture Sci 48:199–207 (in Japanese with English abstract)
Yokokawa K, Taniguchi N (1988) Morphological and genetic differences between artificial bred seeds and natural bred seeds of red sea bream for Pen-net culture. Bull Kagawa Pref Fish Exp Stn 3:31–42 (in Japanese with English abstract)
Miyashita S, Murata O, Sawada Y, Okada T, Kubo Y, Ishitani Y, Seoka M, Kumai H (2000) Maturation and spawning of cultured bluefin tuna, Thunnus thynnus. Aquacult Sci 48:475–488 (in Japanese with English abstract)
Takashi T, Kohno H, Sakamoto W, Miyashita S, Murata O, Sawada Y (2006) Diel and ontogenetic body density change in Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquacult Res 37:1172–1179
Sakamoto W, Okamoto K, Uehabu T, Kato k, Murata O (2005) Specific gravity change of bluefin tuna larvae. Nippon Suisan Gakkaishi 71:80–82 (in Japanese)
Taniguchi N, Azuma K, Umeda S (1988) Significant difference in growth and survival rate among sib-groups of red sea bream marked by isozyme gene. Nippon Suisan Gakkaishi 54:553–557 (in Japanese with English abstract)
Osawa Y, Machida H, Tsutsui K, Kajishima T (1984) Gene analaysis on the progenies of gynogenetic triploid ginbuna Carassius auratus langsdorfii. J Fac Sci Shinshu Univ 19:27–35 (in Japanese)
Zhang F, Oshiro T, Takashima F (1992) Chromosome synapsis and recombination during meiotic division in gynogenetic triploid ginbuna, Carassius auratus langsdorfii. Ichthyol Res 39:151–155
Acknowledgments
We are grateful to the anonymous reviewers for their constructive comments on the earlier version of the manuscript. We thank Eri Tomioka for working on mtDNA analysis and Eiichi Kawakami for collecting PBT fertilized eggs. We also are grateful to staff of Amami Station, RCTA, SNF, FRA, for their support. Part of this study was financially supported by the Research Project for Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries (1905), Ministry of Agriculture, Forestry and Fisheries, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takebe, T., Kurihara, T., Suzuki, N. et al. Onset of individual growth difference in larviculture of Pacific bluefin tuna Thunnus orientalis using fertilized eggs obtained from one female. Fish Sci 78, 343–350 (2012). https://doi.org/10.1007/s12562-011-0449-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0449-1