Abstract
Background
Because procarbazine is not available in the mainland of China, a risk-adapted chemotherapy without the drug was adopted for children with Hodgkin lymphoma (HL) in two tertiary referral centers for childhood cancer in Shanghai. The objective of the present study was to obtain the results comparable with those of previous studies.
Methods
From January 1998 to December 2009, patients below 18 years with newly diagnosed, untreated HL were enrolled in the study. The patients were stratified into risk groups R1 (early stage), R2 (intermediate stage) and R3 (advanced stage). All the patients who had attained a complete remission were not given involved field radiotherapy.
Results
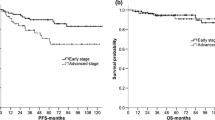
Fifty-six patients were eligible for the study. The 4-year event-free survival (EFS) rate was 100%, 80.3%±7.2%, and 62.5%±12.1% for the risk groups R1, R2, and R3, respectively. There was statistically significant difference in EFS between patients with and those without B symptoms (P<0.001). In group R2, the EFS rate was higher for patients treated with chemotherapy combined with radiation (100% vs. 75%±8.8%). But no statistical difference was observed (P=0.177). At the time of evaluation (December 31, 2010), secondary malignancy was not observed.
Conclusions
A significant fraction of children with early stage or intermediate stage HL can be cured with a chemotherapy regimen without procarbazine. Complete response to chemotherapy seems not to be a determinant to omit radiotherapy.
Similar content being viewed by others
References
Liang RHS, Yau CC, Muckaden MA, Dinshaw KA, Todd D. Hodgkin’s disease in Asia. In: Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM, eds. Hodgkin’s Disease. Philadelphia: Lippincott Williams & Williams, 1999: 771–780.
Bao PP, Zheng Y, Wang CF, Gu K, ** F, Lu W. Time trends and characteristics of childhood cancer among children age 0–14 in Shanghai. Pediatr Blood Cancer 2009;53:13–16.
Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol 2002;20:3765–3771.
Lukes R, Butler J. The pathology and nomenclature of Hodgkin’s disease. Cancer Res 1966;26:1063–1083.
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswald meeting. J Clin Oncol 1989;7:1630–1636.
Schwartz CL. The management of Hodgkin disease in the young child. Curr Opin Pediar 2003;15:10–16.
Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma state of the science. Br J Haematol 2009;144:24–40.
Landman-Parker J, Pacquement H, Leblanc T, Habrand JL, Terrier-Lacombe MJ, Bertrand Y, et al. Localized childhood Hodgkin’s disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before lowdose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol 2000;18:1500–1507.
Dorffel W, Luders H, Ruhl U, Albrecht M, Marciniak H, Parwaresch R, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin’s disease in children and adolescents: analysis and outlook. Klin Padiatr 2003;215: 139–145.
Kung FH, Schwartz CL, Ferree CR, London WB, Ternberg JL, Behm FG, et al. POG8625: a randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with stages I, IIA, IIIA1 Hodgkin disease: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol 2006;28:362–368.
Donaldson SS, Link MP, Weinstein HJ, Rai SN, Brain S, Billett AL, et al. Final results of a prospective clinical trial with VAMP and low-dose involved field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol 2007;25:332–337.
Schellong G, Brämswig JH, Hörnig-Franz I, Schwarze EW, Pötter R, Wannenmacher M. Hodgkin’s disease in children: combined modality treatment for stages IA, IIB, and IIA. Results in 356 patients of German/Austrian Pediatric Study Group. Ann Oncol 1994;5(Suppl 2):113–115.
Weiner MA, Leventhal B, Brecher ML, Marcus RB, Cantor A, Gieser PW, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol 1997;15:2769–2779.
Hudson MM, Krasin M, Link MP, Donaldson SS, Billups C, Merchant TE, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004;22:4541–4550.
Rühl U, Albrecht M, Dieckmann K, Lüders H, Marciniak H, Schellenberg D, et al. Response-adapted radiotherapy in the treatment of pediatric Hodgkin’s disease: an interim report at 5 years of the German GPOH-HD95 trial. Int J Radiat Oncol Biol Phys 2001;51:1209–1218.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, YJ., Tang, JY., Pan, C. et al. Risk-adapted chemotherapy without procarbazine in treatment of children with Hodgkin lymphoma. World J Pediatr 9, 32–35 (2013). https://doi.org/10.1007/s12519-012-0390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-012-0390-0