Abstract
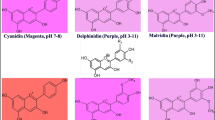
Blue flowers, which are rare, have long been a focus for researchers engaged in colored flower breeding. In this study, Plumbago auriculata, a plant with blue flowers, and its white flower forma Plumbago auriculata f. alba were used as research materials. Flower color quantification, environmental scanning electron microscopy (ESEM) observation of petal epidermal cells, and determination of the cell sap pH were carried out; moreover, metabolomic analysis was used to analyze the flavonoid metabolites of both types of petals. The results showed that the flower color of P. auriculata was bluish violet, and there was no significant difference in the epidermal cell distribution of the white and blue petals. The key substance that determined the bluish violet flower color was cyanidin-3-O-(6″-p-coumaroylglucoside) (Cy3-pC·G). Compared to the white petal cells, the blue petal cells exhibited up to 40 upregulated flavonoid compounds that were copigments. Together with the significantly increased pH (5.92 ± 0.12) of the petal cell sap, these copigments affected the color presentation of Cy3-pC·G, causing the final petal color of P. auriculata to be bluish violet. The results of this study indicate that cyanidin is an important anthocyanidin in blue flowers, which provides new insights into the breeding of blue flowers.









Similar content being viewed by others
Abbreviations
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- Cy3-pC·G:
-
Cyanidin-3-O-(6″-p-coumaroylglucoside)
- ESEM:
-
Environmental scanning electron microscopy
- F3′5′H :
-
Flavonoid 3′,5′-hydroxylase
- F3′H :
-
Flavonoid 3′-monooxygenase
- FC:
-
Fold change
- FFA:
-
Formalin-acetic acid-alcohol
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- PCA:
-
Principal component analysis
- QC:
-
Quality control
- UPLC–MS/MS:
-
Ultraperformance liquid chromatography-tandem mass spectrometry
- VIP:
-
Variable importance in projection
References
Baumann K, Perez-Rodriguez M, Bradley D, Venail J, Bailey P, ** H, Martin C (2007) Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development 134(9):1691–1701
Brouillard R (1982) Chemical structure of anthocyanins, vol 1. Academic Press, New York
Brouillard R (1983) The in vivo expression of anthocyanin colour in plants. Phytochemistry 22(6):1311–1323
Brouillard R, Dubois JE (1977) Mechanism of the structural transformations of anthocyanins in acidic media. J Am Chem Soc 99(5):1359–1364
Brouillard R, Delaporte B, Dubois JE (1978) Chemistry of anthocyanin pigments. 3. Relaxation amplitudes in pH-jump experiments. J Am Chem Soc 100(19):6202–6205
Castañeda-Ovando A, de Lourdes Pacheco-Hernández M, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113(4):859–871
Chandler S, Tanaka Y (2007) Genetic modification in floriculture. Crit Rev Plant Sci 26(4):169–197
Cheminat A, Brouillard R (1986) PMR investigation of 3-O-(β-d-glucosyl) malvidin structural transformations in aqueous solutions. Tetrahedron Lett 27(37):4457–4460
Chen LJ, Hrazdina G (1981) Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry 20(2):297–303
Dyer AG, Whitney HM, Arnold SE, Glover BJ, Chittka L (2007) Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod Plant Interact 1(1):45–55
Farr JE, Sigurdson GT, Giusti MM (2018) Influence of cyanidin glycosylation patterns on carboxypyranoanthocyanin formation. Food Chem 259:261–269
Forkmann G (1991) Flavonoids as flower pigments: the formation of the natural spectrum and its extension by genetic engineering. Plant Breed 106(1):1–26
Gonnet JF (1999) Colour effects of co-pigmentation of anthocyanins revisited—2. A colorimetric look at the solutions of cyanin co-pigmented byrutin using the CIELAB scale. Food Chem 66(3):387–394
Gorton HL, Vogelmann TC (1996) Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiol 112(3):879–888
Hernandez LF, Larsen AO (2013) Visual definition of physiological maturity in sunflower (Helianthus annuus L.) is associated with receptacle quantitative color parameters. Span J Agric Res 2:447–454
Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7(7):1071
Holton TA, Brugliera F, Tanaka Y (1993) Cloning and expression of flavonol synthase from Petunia hybrida. Plant J 4(6):1003–1010
Hoshino T, Matsumoto U, Goto T (1981) Self-association of some anthocyanins in neutral aqueous solution. Phytochemistry 20(8):1971–1976
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28(1):27–30
Khoo HE, Azlan A, Tang ST, Lim SM (2017) Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 61(1):1–21
Li W, Gao S, Li Q, Shen P, Li Y, Hu D, Li J (2020) Transcriptome profiling of Plumbago auriculata Lam. in response to cold stress. Acta Physiol Plant 42:1–18
Lou Q, Liu Y, Qi Y, Jiao S, Tian F, Jiang L, Wang Y (2014) Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J Exp Bot 65(12):3157–3164
Mazza G, Brouillard R (1990) The mechanism of co-pigmentation of anthocyanins in aqueous solutions. Phytochemistry 29(4):1097–1102
Mazza G, Miniati E (1993) Grapes. Anthocyanins in fruits, vegetables and grains. CRC Press, Boca Raton, pp 149–199
Mizuno T, Uehara A, Mizuta D, Yabuya T, Iwashina T (2015) Contribution of anthocyanin–flavone co-pigmentation to grayed violet flower color of Dutch iris cultivar ‘Tiger’s Eye’under the presence of carotenoids. Sci Hortic 186:201–206
Mol J, Cornish E, Mason J, Koes R (1999) Novel coloured flowers. Curr Opin Biotechnol 10(2):198–201
Noda KI, Glover BJ, Linstead P, Martin C (1994) Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 369(6482):661–664
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181(3):219–229
Rakić V, Rinnan Å, Polak T, Skrt M, Miljković M, Ulrih NP (2019) pH-induced structural forms of cyanidin and cyanidin 3-O-β-glucopyranoside. Dyes Pigm 165:71–80
Shrestha B, Pandey RP, Darsandhari S, Parajuli P, Sohng JK (2019) Combinatorial approach for improved cyanidin 3-O-glucoside production in Escherichia coli. Microb Cell Fact 18(1):7
Stewart RN, Norris KH, Asen S (1975) Microspectrophotometric measurement of pH and pH effect on color of petal epidermal cells. Phytochemistry 14(4):937–942
Tanaka Y, Katsumoto Y, Brugliera F, Mason J (2005) Genetic engineering in floriculture. Plant Cell Tissue Organ Cult 80(1):1–24
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54(4):733–749
Whang SS, Um WS, Song IJ, Lim PO, Choi K, Park KW, Koo JC (2011) Molecular analysis of anthocyanin biosynthetic genes and control of flower coloration by flavonoid 3′, 5′-hydroxylase (F3′ 5′ H) in Dendrobium moniliforme. J Plant Biol 54(3):209–218
Whitney HM, Glover BJ (2007) Morphology and development of floral features recognised by pollinators. Arthropod Plant Interact 1(3):147–158
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126(2):485–493
Xu W, Grain D, Le Gourrierec J, Harscoët E, Berger A, Jauvion V, Salsac F (2013) Regulation of flavonoid biosynthesis involves an unexpected complex transcriptional regulation of TT 8 expression, in A rabidopsis. New Phytol 198(1):59–70
Acknowledgements
This research was supported by Yuanxiang Hang from Sichuan Tianyi Ecological Garden Group Co. Ltd.
Funding
This work was supported by the Sichuan Science and Technology Program (2016NYZ0038).
Author information
Authors and Affiliations
Contributions
Data curation, YL; formal analysis, WL and DH; methodology, YL and SG; project administration, SG; validation, TL; writing–original draft, WL; writing–review and editing, JL and PS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Li, W., Hu, D. et al. Metabolomics Analysis Reveals the Role of Cyanidin Metabolism in Plumbago auriculata Flower Color. J. Plant Biol. 64, 253–265 (2021). https://doi.org/10.1007/s12374-021-09305-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-021-09305-6