Abstract
Introduction
Hair loss is driven by multiple factors, including genetics. Androgenetic alopecia (AGA) is a condition in which treatments necessitate prolonged compliance with prescribed medications. We have developed IVL3001, a long-acting injectable (LAI) formulation of finasteride encapsulated within poly lactic-co-glycolic acid microspheres, to enhance the efficacy of the finasteride and to achieve consistent positive outcomes in adults. An open-label, sequential, single-dose phase I clinical trial was designed to evaluate the safety, pharmacokinetic (PK), and pharmacodynamic (PD) of IVL3001.
Methods
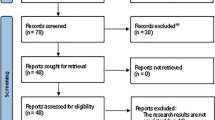
A total of 40 non-smoking, healthy adult males were divided into three cohorts where the IVL3001 group received a single subcutaneous injection of 12–36 mg IVL3001 and 1 mg finasteride (Propecia®) once daily for 28 days. The plasma concentrations of finasteride, dihydrotestosterone (DHT), and testosterone were measured using liquid chromatography–tandem mass spectrometry. The tolerability of the injections was assessed, and compartment models were developed to predict the effective dose and assess PK/PD profiles.
Results
IVL3001 and finasteride 1 mg tablets were well tolerated. IVL3001 showed consistent plasma concentrations without bursts or fluctuations. Consistent with its mechanism of action, IVL3001 reduced DHT levels. Simulation data showed that the administration of 12–36 mg of IVL3001 every 4 weeks achieved plasma concentrations similar to finasteride, with comparable DHT reduction.
Conclusion
The present study represents the first clinical trial to evaluate the safety, pharmacokinetic (PK), pharmacodynamic (PD), and tolerability of finasteride long-acting injectables (LAI) in adults. The rapid onset of action sustained effective drug concentration and the prolonged half-life of IVL3001 suggest that it offers multiple benefits over conventional oral formulations in terms of therapeutic response and compliance.
Trial Registration
ClinicalTrials.gov identifier, NCT04945226.






Similar content being viewed by others
Data Availability
The datasets acquired and examined in the present investigation are not publicly available due to confidentiality considerations.
References
Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005;80(10):1316–22.
Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247–54.
Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001 Mar-Apr;19(2):161–6.
Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317(7162):865–9.
Alfonso M, Richter-Appelt H, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21(11):1829–36.
Han SH, Byun JW, Lee WS, Kang H, Kye YC, Kim KH, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012;24(3):311–8.
Bingham KD, Shaw DA. The metabolism of testosterone by human male scalp skin. J Endocrinol. 1973;57(1):111–21.
Yun S-I, Lee S-K, Goh E-A, Kwon OS, Choi W, Kim J, et al. Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia. Sci Rep. 2022;12(1):1607.
Dhurat R, Sharma A, Rudnicka L, Kroumpouzos G, Kassir M, Galadari H, et al. 5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatol Ther. 2020;33(3):e13379.
Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008;5(12):2917–24.
Mysore V, Shashikumar BM. Guidelines on the use of finasteride in androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2016 Mar-Apr;82(2):128–34.
Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). J Investig Dermatol Symp Proc. 2003;8(1):20–3.
Zito PM, Bristas KG, Syed K. Finasteride. StatPearls Publishing; 2022.
Kim JH, Na J, Bak DH, Lee BC, Lee E, Choi MJ, et al. Development of finasteride polymer microspheres for systemic application in androgenic alopecia. Int J Mol Med. 2019;43(6):2409–19.
Hosking AM, Juhasz M, Atanaskova MN. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019;5(2):72–89.
Brissos S, Veguilla MR, Taylor D, Balanza-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219.
Zolezzi M, Abouelhassan R, Eltorki Y, Haddad PM, Noorizadeh M. Long-Acting Injectable Antipsychotics: A Systematic Review of Their Non-Systemic Adverse Effect Profile. Neuropsychiatr Dis Treat. 2021;17:1917–26.
Tuntland T, Ethell B, Kosaka T, Blasco F, Zang RX, Jain M, et al. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front Pharmacol. 2014;5:174.
McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs. 1999;57(1):111–26.
Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30(12):2563–71.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45.
Bossie CA, Alphs LD, Correll CU. Long-acting injectable versus daily oral antipsychotic treatment trials in schizophrenia: pragmatic versus explanatory study designs. International Clinical Psychopharmacology. 2015;30(5).
Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013 Aug;66(8 Suppl):S37–41.
Poloni N, Ielmini M, Caselli I, Lucca G, Gasparini A, Gasparini A, et al. Oral Antipsychotic Versus Long-Acting Injections Antipsychotic in Schizophrenia Spectrum Disorder: a Mirror Analysis in a Real-World Clinical Setting. Psychopharmacol Bull. 2019;49(2):17–27.
Hughes DA. Estimation of the impact of noncompliance on pharmacokinetics: an analysis of the influence of dosing regimens. Br J Clin Phrmacol. 2008;65(6):871–8.
Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(16):18–23.
Macdougall IC, Padhi D, Jang G. Pharmacology of darbepoetin alfa. Nephrology Dialysis Transplantation. 2007;22(suppl_4):iv2-iv9.
Li Z, Radin A, Li M, Hamilton JD, Kajiwara M, Davis JD, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of dupilumab in healthy adult subjects. Clin Pharmacol drug Dev. 2020;9(6):742–55.
Carlin JR, Höglund P, Eriksson L, Christofalo P, Gregoire SL, Taylor AM, et al. Disposition and pharmacokinetics of [14C] finasteride after oral administration in humans. Drug Metab Dispos. 1992;20(2):148–55.
Ohtawa M, Morikawa H, Shimazaki J. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide, a new type of specific competitive inhibitor of testosterone 5α-reductase, in volunteers. Eur J Drug Metab Pharmacokinet. 1991;16:15–21.
Le T, Aguilar B, Mangal JL, Acharya AP. Oral drug delivery for immunoengineering. Bioeng Transl Med. 2022;7(1): e10243.
Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145(3):385–405.
McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs. 1999;57(1):111–26.
Steiner JF. Clinical pharmacokinetics and pharmacodynamics of finasteride. Clin Pharmacokinet. 1996;30(1):16–27.
Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011;10(5):328–9.
Lim HS. Evolving role of modeling and simulation in drug development. Transl Clin Pharmacol. 2019;27(1):19–23.
Day RO, Williams KM. Open-label extension studies. Drug Saf. 2007;30(2):93–105.
Kahan BC, Cro S, Doré CJ, Bratton DJ, Rehal S, Maskell NA, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15(1):456.
Trone JC, Ollier E, Chapelle C, Bertoletti L, Cucherat M, Mismetti P, et al. Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis. Anna Oncol. 2018;29(4):803–11.
Paton C, Craig TKJ, McConnell B, Barnes TRE. Side-effect monitoring of continuing LAI antipsychotic medication in UK adult mental health services. Ther Adv Psychopharmacol. 2021;11:2045125321991278.
Haddad P, Fleischhacker WW. 47 Adverse effects and antipsychotic long-acting injections. In: Haddad P, Lambert T, Lauriello J, editors. Antipsychotic long-acting injections. Oxford: Oxford University Press; 2010.
Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8(6):1747–53.
Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J. 2012;3(1):62–5.
Acknowledgements
The authors thank the principal investigators and trial-site staff of Nucleus Network Pty Ltd, Brisbane, Australia. We thank the participants who generously agreed to participate in this clinical trial. We thank the Inventage Lab editorial department for their help with editing and formatting.
Funding
This study was supported by Inventage Lab, Seoul, Republic of Korea. The study rapid services fee, and open access fees were funded by Inventage Lab, Seoul, Republic of Korea. The study sponsor was involved in several aspects of the research, including experimental design, analysis, and interpretation of data, and drafting of manuscript.
Author information
Authors and Affiliations
Contributions
Inventage Lab research team conceived and designed the study. Heesun Kim and Juhee Kim participated in the trial inspection. Kyeong-Ryoon Lee and Choongho Ryu were involved in statistical analysis. Heesun Kim and Kyeong-Ryoon Lee. prepared the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the clinical trial is related to drugs with commercial significance, and no conflicts of interest may influence the objectivity and integrity of the study process.
Ethical Approval
This study was conducted according to the Therapeutic Goods Administration (TGA) and Human Research Ethics Committee (HREC) guidelines. The whole study was conducted in accordance with the provisions of the Helsinki Declaration and the standards for clinical trial management (GCP), and the protocol was registered at www.clinicaltrials.gov (NCT04945226; Reg date 2021–06-30). Potential volunteers completed signed informed consent and underwent a screening test prior to the trial.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Ryu, C., Lee, M. et al. A Phase I, Open-Label, Sequential, Single-Dose Clinical Trial to Evaluate the Pharmacokinetic, Pharmacodynamic, and Safety of IVL3001, a Finasteride-Based Novel Long-Acting Injection for Androgenetic Alopecia. Adv Ther 41, 2936–2952 (2024). https://doi.org/10.1007/s12325-024-02890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02890-1