Abstract
Introduction
Apnoea of prematurity (AOP) is among the most common diagnoses in the neonatal intensive care unit. Caffeine treatment is a preferred treatment choice. However, neonatal caffeine therapy results in significant intersubject variability. This study aimed to determine the effects of plasma caffeine levels based on standard dose and genetic variability on clinical response to caffeine citrate in Chinese preterm infants.
Methods
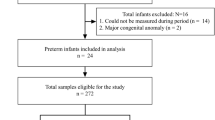
This single-center and retrospective study examined data from 112 preterm infants (< 35 weeks gestational age) between July 2017 and July 2018. Subjects were divided into apnoea-free (n = 48) and apnoeic (n = 64) groups, and their clinical outcomes were summarized. Liquid chromatography-tandem mass spectrometry was used to measure levels of caffeine and its primary metabolites. Eighty-eight single-nucleotide polymorphisms were chosen for genoty** by a MassARRAY system.
Results
Preterm infants in the apnoea-free group were associated with a reduction in the incidence of bronchopulmonary dysplasia and a reduced requirement for patent ductus arteriosus ligation. No significant association was observed between plasma-trough-concentration-to-dose (C0/D) ratio and birth weight, gestational age, or postnatal age in either group. Polymorphisms in CYP1A2 and aryl hydrocarbon receptor (AHR) genes did not affect plasma caffeine levels. Polymorphisms in adenosine receptor genes ADORA1 (rs10920568 and rs12744240), ADORA2A (rs34923252 and rs5996696), and ADORA3 (rs10776727 and rs2298191), especially in AHR (rs4410790) and adenosine deaminase (rs521704), play critical roles in the interindividual response to caffeine therapy.
Conclusions
Genetic polymorphisms in caffeine’s target receptors, but not the exposure levels based on the standard dosing, were associated with variable responses to caffeine therapy in preterm neonates. Future studies are needed to uncover how these genetic variants affect responses to caffeine therapy in this patient population.



Similar content being viewed by others
References
Eichenwald EC, F. Committee on A.A.o.P. Newborn, apnea of prematurity. Pediatrics. 2016;137(1):e20153757.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, G. Caffeine for Apnea of Prematurity Trial. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, G. Caffeine for Apnea of Prematurity Trial. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–902.
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A, Ohlsson A, Barrington KJ, Roberts RS, I. Caffeine for Apnea of Prematurity Trial. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307(3):275–82.
Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG, Dewey D, D’Ilario J, Doyle LW, Grunau RE, Moddemann D, Nelson H, Ohlsson A, Solimano A, Tin W, G. Caffeine for Apnea of Prematurity Trial. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 2017;171(6):564–72.
Murner-Lavanchy IM, Doyle LW, Schmidt B, Roberts RS, Asztalos EV, Costantini L, Davis PG, Dewey D, D'Ilario J, Grunau RE, Moddemann D, Nelson H, Ohlsson A, Solimano A, Tin W, Anderson PJ, G. Caffeine for Apnea of Prematurity Trial. Neurobehavioral outcomes 11 years after neonatal caffeine therapy for apnea of prematurity. Pediatrics. 2018;141(5):e20174047.
Lodha A, Entz R, Synnes A, Creighton D, Yusuf K, Lapointe A, Yang J, Shah PS, N. investigators of the Canadian Neonatal, N. the Canadian Neonatal Follow-up. Early caffeine administration and neurodevelopmental outcomes in preterm infants. Pediatrics. 2019;143(1):e20181348.
Lodha A, Seshia M, McMillan DD, Barrington K, Yang J, Lee SK, Shah PS, N. Canadian Neonatal. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015;169(1):33–8.
Bloch-Salisbury E, Hall MH, Sharma P, Boyd T, Bednarek F, Paydarfar D. Heritability of apnea of prematurity: a retrospective twin study. Pediatrics. 2010;126(4):e779–87.
Kumral A, Tuzun F, Yesilirmak DC, Duman N, Ozkan H. Genetic basis of apnoea of prematurity and caffeine treatment response: role of adenosine receptor polymorphisms: genetic basis of apnoea of prematurity. Acta Paediatr. 2012;101(7):e299-303.
Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, Nasef N. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr. 2015;174(7):949–56.
Koch G, Datta AN, Jost K, Schulzke SM, van den Anker J, Pfister M. Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm neonates. J Pediatr. 2017;191:50–6 ((e1)).
F. Committee on, Newborn P. American Academy of, Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111(4 Pt 1):914–7.
Lodha A, Zhu Q, Lee SK, Shah PS, N. Canadian Neonatal. Neonatal outcomes of preterm infants in breech presentation according to mode of birth in Canadian NICUs. Postgrad Med J. 2011;87(1025):175–9.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92(4):529–34.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. 1986;33(1):179–201.
Chen F, Hu ZY, Parker RB, Laizure SC. Measurement of caffeine and its three primary metabolites in human plasma by HPLC-ESI-MS/MS and clinical application. Biomed Chromatogr. 2017; 31(6). https://doi.org/10.1002/bmc.3900.
Jia W, Li J, Du F, Sun Y, Xu F, Wang F, Olaleye OE, Chen D, Tang W, Zuo J, Li C. Assay development for determination of DZ2002, a new reversible SAHH inhibitor, and its acid metabolite DZA in blood and application to rat pharmacokinetic study. J Pharm Anal. 2019;9(1):25–33.
Taha D, Kirkby S, Nawab U, Dysart KC, Genen L, Greenspan JS, Aghai ZH. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J Matern Fetal Neonatal Med. 2014;27(16):1698–702.
Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23(4):278–85.
Cox C, Hashem NG, Tebbs J, Bookstaver PB, Iskersky V. Evaluation of caffeine and the development of necrotizing enterocolitis. J Neonatal Perinatal Med. 2015;8(4):339–47.
Lampkin SJ, Turner AM, Lakshminrusimha S, Mathew B, Brown J, Fominaya CE, Johnson KK. Association between caffeine citrate exposure and necrotizing enterocolitis in preterm infants. Am J Health Syst Pharm. 2013;70(7):603–8.
Shrestha B, Jawa G. Caffeine citrate––Is it a silver bullet in neonatology? Pediatr Neonatol. 2017;58(5):391–7.
Natarajan G, Botica ML, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exercise? Pediatrics. 2007;119(5):936–40.
Puia-Dumitrescu M, Smith PB, Zhao J, Soriano A, Payne EH, Harper B, Bendel-Stenzel E, Moya F, Chhabra R, Ku L, Laughon M, Wade KC, C. Best Pharmaceuticals for Children Act-Pediatric Trials Network Steering. Dosing and safety of off-label use of caffeine citrate in premature infants. J Pediatr. 2019;211:27–32 ((e1)).
Thorn CF, Aklillu E, McDonagh EM, Klein TE, Altman RB. PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012;22(5):389–95.
Song G, Sun X, Hines RN, McCarver DG, Lake BG, Osimitz TG, Creek MR, Clewell HJ, Yoon M. Determination of human hepatic CYP2C8 and CYP1A2 age-dependent expression to support human health risk assessment for early ages. Drug Metab Dispos. 2017;45(5):468–75.
McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312.
Kumar VHS, Lipshultz SE. Caffeine and clinical outcomes in premature neonates. Children (Basel). 2019;6(11):118.
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98(3):1591–625.
Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–86.
Nordestgaard AT, Nordestgaard BG. Coffee intake, cardiovascular disease and all-cause mortality: observational and Mendelian randomization analyses in 95,000–223,000 individuals. Int J Epidemiol. 2016;45(6):1938–52.
Josse AR, Da Costa LA, Campos H, El-Sohemy A. Associations between polymorphisms in the AHR and CYP1A1-CYP1A2 gene regions and habitual caffeine consumption. Am J Clin Nutr. 2012;96(3):665–71.
Ziv-Gal A, Flaws JA, Mahoney MM, Miller SR, Zacur HA, Gallicchio L. Genetic polymorphisms in the aryl hydrocarbon receptor-signaling pathway and sleep disturbances in middle-aged women. Sleep Med. 2013;14(9):883–7.
Shivanna B, Maity S, Zhang S, Patel A, Jiang W, Wang L, Welty SE, Belmont J, Coarfa C, Moorthy B. Gene expression profiling identifies cell proliferation and inflammation as the predominant pathways regulated by aryl hydrocarbon receptor in primary human fetal lung cells exposed to hyperoxia. Toxicol Sci. 2016;152(1):155–68.
Sauzeau V, Carvajal-Gonzalez JM, Riolobos AS, Sevilla MA, Menacho-Marquez M, Roman AC, Abad A, Montero MJ, Fernandez-Salguero P, Bustelo XR. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem. 2011;286(4):2896–909.
Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA. 2005;102(43):15676–81.
Mazzotti DR, Guindalini C, Pellegrino R, Barrueco KF, Santos-Silva R, Bittencourt LR, Tufik S. Effects of the adenosine deaminase polymorphism and caffeine intake on sleep parameters in a large population sample. Sleep. 2011;34(3):399–402.
Acknowledgements
Funding
This research was supported by the Scientific Research Foundation for Young Scholars at the Children’s Hospital of Nan**g Medical University (ETYYQM2014024), the Specially Appointed Medical Expert Project of Jiangsu Commission of Health (2019) and Special fund for clinical research of Wu Jie** Medical Foundation (320.6750.2020-04-07). This study was also supported by Nan**g Medical University Science and Technology Development Foundation (NMUB2019195). The journal’s Rapid Service Fees for this article were funded by the Specially Appointed Medical Expert Project of Jiangsu Commission of Health (2019).
Medical Writing Assistance
Medical writing support, under the guidance of the authors, was provided by Elsevier's WebShop, a company registered in Kidlington (UK). The medical writing assistance fee was funded by Nan**g Medical University Scienceand Technology Development Foundation (NMUB2019195).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
**n He, **-Chun Qiu, Ke-Yu Lu, Feng Chen, Rui Cheng: Principal investigators who designed the study, analysed the data, and wrote the paper. **n He, Ke-Yu Lu, Hong-Li Guo: Performed the data collection and analysis. Wei-Wei Jia: Performed the caffeine assay. Ling Li, Ming-Ming Ni, Yun Liu, **g Xu: Assisted with the study and data analysis. Feng Chen, **n He, Ling Li, Hong-Li Guo: Wrote the paper. **n He, **-Chun Qiu and Ke-Yu Lu contributed equally to this work.
Disclosures
**n He, **-Chun Qiu, Ke-Yu Lu, Hong-Li Guo, Ling Li, Wei-Wei Jia, Ming-Ming Ni, Yun Liu, **g Xu, Feng Chen and Rui Cheng have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Helsinki Declaration and the study protocol was approved by the Children’s Hospital of Nan**g Medical University ethics committee (Protocol number 201902082-1). The informed consent was obtained from a parent of each infant.
Data Availability
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, X., Qiu, JC., Lu, KY. et al. Therapy for Apnoea of Prematurity: A Retrospective Study on Effects of Standard Dose and Genetic Variability on Clinical Response to Caffeine Citrate in Chinese Preterm Infants. Adv Ther 38, 607–626 (2021). https://doi.org/10.1007/s12325-020-01544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01544-2