Abstract
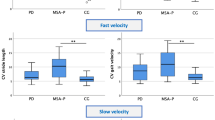
Given the high morbidity related to the progression of gait deficits in spinocerebellar ataxias (SCA), there is a growing interest in identifying biomarkers that can guide early diagnosis and rehabilitation. Spatiotemporal parameter (STP) gait analysis using inertial measurement units (IMUs) has been increasingly studied in this context. This study evaluated STP profiles in SCA types 3 and 10, compared them to controls, and correlated them with clinical scales. IMU portable sensors were used to measure STPs under four gait conditions: self-selected pace (SSP), fast pace (FP), fast pace checking-boxes (FPCB), and fast pace with serial seven subtractions (FPS7). Compared to healthy subjects, both SCA groups had higher values for step time, variability, and swing time, with lower values for gait speed, cadence, and step length. We also found a reduction in speed gain capacity in both SCA groups compared to controls and an increase in speed dual-task cost in the SCA10 group. However, there were no significant differences between the SCA groups. Swing time, mean speed, and step length were correlated with disease severity, risk of falling and functionality in both clinical groups. In the SCA3 group, fear of falling was correlated with cadence. In the SCA10 group, results of the Montreal cognitive assessment test were correlated with step time, mean speed, and step length. These results show that individuals with SCA3 and SCA10 present a highly variable, short-stepped, slow gait pattern compared to healthy subjects, and their gait quality worsened with a fast pace and dual-task involvement.

Similar content being viewed by others
References
Schöggl J, Siegert S, Boltshauser E, Freilinger M, Schmidt WM. A De Novo Missense NPTX1 Variant in an Individual with Infantile-Onset Cerebellar Ataxia. Mov Disord. 2022;37:1774–6.
Coutelier M, Jacoupy M, Janer A, Renaud F, Auger N, Saripella GV, et al. NPTX1 mutations trigger endoplasmic reticulum stress and cause autosomal dominant cerebellar ataxia. Brain. 2022;145:1519–34.
De Castilhos RM, Furtado GV, Gheno TC, Schaeffer P, Russo A, Barsottini O, et al. Spinocerebellar ataxias in Brazil - Frequencies and modulating effects of related genes. Cerebellum. 2014;13:17–28.
Takiyama Y, Nishizawa M, Tanaka H, Kawashima S, Sakamoto H, Karube Y, et al. The gene for Machado–Joseph disease maps to human chromosome 14q. Nat Genet. 1993;4:300–4.
Stevanin G, Cancel G, Durr A, Chneiweiss H, Dubourg O, Weissenbach J, et al. The gene for spinal cerebellar ataxia 3 (SCA3) is located in a region of ~3 cM on chromosome 14q24.3-q32.2. Am J Hum Genet. 1995;56:193–201.
Moro A, Munhoz RP, Arruda WO, Raskin S, Moscovich M, Teive HAG. Spinocerebellar ataxia type 3: Subphenotypes in a cohort of brazilian patients. Arq Neuropsiquiatr. 2014;72:659–62.
Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: A 2-year follow-up study. Neurology. 2011;77:1035–41.
Matsuura T, Fang P, Pearson CE, Jayakar P, Ashizawa T, Roa BB, et al. Interruptions in the expanded ATTCT repeat of spinocerebellar ataxia type 10: Repeat purity as a disease modifier? Am J Hum Genet. 2006;78:125–9.
Raskin S, Ashizawa T, Teive HAG, Arruda WO, Fang P, Gao R, et al. Reduced penetrance in a Brazilian family with spinocerebellar ataxia type 10. Arch Neurol. 2007;64:591–4.
Teive HAG, Ashizawa T. Spinocerebellar ataxia type 10: From amerindians to latin americans. Curr Neurol Neurosci Rep. 2013;13:9–11.
Rasmussen A, Matsuura T, Ruano L, Yescas P, Ochoa A, Ashizawa T, et al. Clinical and genetic analysis of four Mexican families with spinocerebellar ataxia type 10. Ann Neurol. 2001;50:234–9.
Matsuura T, Ranum LPW, Volpini V, Pandolfo M, Sasaki H, Tashiro K, et al. Spinocerebellar ataxia type 10 is rare in populations other than Mexicans. Neurology. 2002;58:983–4.
Gheno TC, Furtado GV, Saute JAM, Donis KC, Fontanari AMV, Emmel VE, et al. Spinocerebellar ataxia type 10: common haplotype and disease progression rate in Peru and Brazil. Eur J Neurol. 2017;24:892–e36.
Véliz-Otani D, Cubas-Montecino D, Milla-Neyra K, Ashizawa T, Saraiva-Pereira ML, Jardim LB, et al. Response to ATXN10 Microsatellite Distribution in a Peruvian Amerindian Population. Cerebellum. 2021;20:946–7.
Teive HAG, Munhoz RP, Raskin S, Arruda WO, de Paola L, Werneck LC, et al. Spinocerebellar ataxia type 10: Frequency of epilepsy in a large sample of Brazilian patients. Mov Disord. 2010;25:2875–8.
Ashizawa T, **a G. Ataxia Articles from Continuum : Lifelong Learning in Neurology are provided here courtesy of American Academy of Neurology. Contin Mineap Minn. 2016;22:1208–26.
Matsushima A, Yoshida K, Genno H, Murata A, Matsuzawa S, Nakamura K, et al. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias. 2015;2:1–7.
Buckley E, Mazzà C, McNeill A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture. 2018;60:154–63.
Geritz J, Maetzold S, Steffen M, Pilotto A, Corrà MF, Moscovich M, et al. Motor, cognitive and mobility deficits in 1000 geriatric patients: protocol of a quantitative observational study before and after routine clinical geriatric treatment - The ComOn-study. BMC Geriatr. 2020;20:1–13.
Donath L, Faude O, Lichtenstein E, Nuesch C, Mündermann A. Validity and reliability of a portable gait analysis system for measuring spatiotemporal gait characteristics: Comparison to an instrumented treadmill. J NeuroEngineering Rehabil. 2016;13:1–9.
Nüesch C, Roos E, Pagenstert G, Mündermann A. Measuring joint kinematics of treadmill walking and running: Comparison between an inertial sensor based system and a camera-based system. J Biomech. 2017;57:32–8. https://doi.org/10.1016/j.jbiomech.2017.03.015.
Bettecken K, Bernhard F, Sartor J, Hobert MA, Hofmann M, Gladow T, et al. No relevant association of kinematic gait parameters with Health-related Quality of Life in Parkinson’s disease. PLoS ONE. 2017;12:1–11.
Pham MH, Warmerdam E, Elshehabi M, Schlenstedt C, Bergeest LM, Heller M, et al. Validation of a lower back “wearable”-based sit-to-stand and stand-to-sit algorithm for patients with Parkinson’s disease and older adults in a home-like environment. Front Neurol. 2018;9:1–11.
Pham MH, Elshehabi M, Haertner L, Del Din S, Srulijes K, Heger T, et al. Validation of a step detection algorithm during straight walking and turning in Patients with Parkinson’s disease and older adults using an inertial measurement unit at the lower back. Front Neurol. 2017;8:1–9.
Metzger FG, Hobert MA, Ehlis AC, Hasmann SE, Hahn T, Eschweiler GW, et al. Dual tasking for the differentiation between depression and mild cognitive impairment. Front Aging Neurosci. 2016;8:1–9.
Hobert MA, Meyer SI, Hasmann SE, Metzger FG, Suenkel U, Eschweiler GW, et al. Gait is associated with cognitive flexibility: A dual-tasking study in healthy older people. Front Aging Neurosci. 2017;9:1–9.
Salkovic D, Hobert MA, Bellut C, Funer F, Renno S, Haertner L, et al. Evidence for a Selectively Regulated Prioritization Shift Depending on Walking Situations in Older Adults. Front Aging Neurosci. 2017;9:1–9.
Del Din S, Elshehabi M, Galna B, Hobert MA, Warmerdam E, Suenkel U, et al. Gait analysis with wearables predicts conversion to parkinson disease. Ann Neurol. 2019;86:357–67.
Kressig RW, Beauchet O, Gaitrite E, Group N. Guidelines for clinical applications of spatio-temporal. Aging Clin Exp Res. 2005;18:174–6.
Bock O, Engelhard K, Guardiera P, Allmer H, Kleinert J. Gerontechnology and human cognition. IEEE Eng Med Biol Mag. 2008;27:23–8.
Schniepp R, Wuehr M, Neuhaeusser M, Kamenova M, Dimitriadis K, Klopstock T, et al. Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov Disord. 2012;27:125–31.
Braga-Neto P, Godeiro-Junior C, Dutra LA, Pedroso JL, Barsottini OGP. Translation and validation into Brazilian version of the Scale of the Assessment and Rating of Ataxia (SARA). Arq Neuropsiquiatr. 2010;68:228–30.
Camargos FFO, Dias RC, Dias JMD, Freire MTF. Cross-cultural adaptation and evaluation of the psychometric properties of the Falls Efficacy Scale-International Among Elderly Brazilians (FES-I-BRAZIL). Rev Bras Fisioter Sao Carlos Sao Paulo Braz. 2010;14:237–43.
Miyamoto ST, Lombardi I, Berg KO, Ramos LR, Natour J. Brazilian version of the Berg balance scale. Braz J Med Biol Res. 2004;37:1411–21.
Minosso JSM, Amendola F, Alvarenga MRM, de Oliveira MAC. Validação, no Brasil, do Índice de Barthel em idosos atendidos em ambulatórios. Acta Paul Enferm. 2010;23:218–23.
Sarmento ALR. Apresentação e aplicabilidade da versão brasileira da MoCA (Montreal Cognitive Assessment) para rastreio de Comprometimento Cognitivo Leve. Master's thesis, Universidade Federal de São Paulo. 2009.
Serrao M, Pierelli F, Ranavolo A, Draicchio F, Conte C, Don R, et al. Gait pattern in inherited cerebellar ataxias. Cerebellum. 2012;11:194–211.
Bunn LM, Marsden JF, Giunti P, Day BL. Stance instability in spinocerebellar ataxia type 6. Mov Disord. 2013;28:510–6.
Velázquez-Pérez L, Rodriguez-Labrada R, González-Garcés Y, Arrufat-Pie E, Torres-Vega R, Medrano-Montero J, et al. Prodromal Spinocerebellar Ataxia Type 2 Subjects Have Quantifiable Gait and Postural Sway Deficits. Mov Disord. 2021;36:471–80.
Hickey A, Gunn E, Alcock L, Del Din S, Godfrey A, Rochester L, et al. Validity of a wearable accelerometer to quantify gait in spinocerebellar ataxia type 6. Physiol Meas. 2016;37:N105–17.
Palliyath S, Hallett M, Thomas SL, Lebiedowska MK. Gait in patients with cerebellar ataxia. Mov Disord. 1998;13:958–64.
Caliandro P, Serrao M, Padua L, Silvestri G, Iacovelli C, Simbolotti C, et al. Prefrontal cortex as a compensatory network in ataxic gait: A correlation study between cortical activity and gait parameters. Restor Neurol Neurosci. 2015;33:177–87.
Gouelle A, Mégrot F, Presedo A, Husson I, Yelnik A, Penneçot GF. The Gait Variability Index: A new way to quantify fluctuation magnitude of spatiotemporal parameters during gait. Gait Posture. 2013;38:461–5.
Stephenson J, Zesiewicz T, Gooch C, Wecker L, Sullivan K, Jahan I, et al. Gait and balance in adults with Friedreich’s ataxia. Gait Posture. 2015;41:603–7.
Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130:786–98.
Wuehr M, Schniepp R, Ilmberger J, Brandt T, Jahn K. Speed-dependent temporospatial gait variability and long-range correlations in cerebellar ataxia. Gait Posture. 2013;37:214–8.
Milne SC, Hocking DR, Georgiou-Karistianis N, Murphy A, Delatycki MB, Corben LA. Sensitivity of Spatiotemporal Gait Parameters in Measuring Disease Severity in Friedreich Ataxia. Cerebellum. 2014;13:677–88.
Ebersbach G, Sojer M, Valldeoriola F, Wissel J, Müller J, Tolosa E, et al. Comparative analysis of gait in Parkinson’s disease, cerebellar ataxia and subcortical arteriosclerotic encephalopathy. Brain. 1999;122:1349–55.
Ienaga Y, Mitoma H, Kubota K, Morita S, Mizusawa H. Dynamic imbalance in gait ataxia. Characteristics of plantar pressure measurements. J Neurol Sci. 2006;246:53–7.
Matsushima A, Yoshida K, Genno H. Ikeda S ichi. Principal component analysis for ataxic gait using a triaxial accelerometer. J NeuroEngineering Rehabil. 2017;14:1–7.
Rochester L, Galna B, Lord S, Mhiripiri D, Eglon G, Chinnery PF. Gait impairment precedes clinical symptoms in spinocerebellar ataxia type 6. Mov Disord. 2014;29:252–5.
Azadian E, Torbati HRT, Kakhki ARS, Farahpour N. The effect of dual task and executive training on pattern of gait in older adults with balance impairment: A Randomized controlled trial. Arch Gerontol Geriatr. 2016;62:83–9.
Caliandro P, Conte C, Iacovelli C, Tatarelli A, Castiglia SF, Reale G, et al. Exploring risk of falls and dynamic unbalance in cerebellar ataxia by inertial sensor assessment. Sens Switz. 2019;19:1–9.
Bares M, Lungu O, Liu T, Waechter T, Gomez CM, Ashe J. Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res. 2007;180:355–65.
Schniepp R, Wuehr M, Schlick C, Huth S, Pradhan C, Dieterich M, et al. Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol. 2014;261:213–23.
O’Keefe JA, Guan J, Robertson E, Biskis A, Joyce J, Ouyang B, et al. The Effects of Dual Task Cognitive Interference and Fast-Paced Walking on Gait, Turns, and Falls in Men and Women with FXTAS. Cerebellum. 2021;20:212–21.
Sütçü G, Doğan M, Topuz S. Investigation of postural control and spatiotemporal parameters of gait during dual tasks in ataxic individuals. Neurol Sci. 2022;43(10):5943–9.
Jacobi H, Alfes J, Minnerop M, Konczak J, Klockgether T, Timmann D. Dual task effect on postural control in patients with degenerative cerebellar disorders. Cerebellum Ataxias. 2015;2:1–7.
Ilg W, Christensen A, Mueller OM, Goericke SL, Giese MA, Timmann D. Effects of cerebellar lesions on working memory interacting with motor tasks of different complexities. J Neurophysiol. 2013;110:2337–49.
Ilg W, Timmann D. Gait ataxia-specific cerebellar influences and their rehabilitation. Mov Disord. 2013;28:1566–75.
Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, et al. How does explicit prioritization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther. 2010;90(2):177–86.
Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, et al. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–6.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79.
Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250:207–11.
Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felício AC, Minett T, et al. Cerebellar cognitive affective syndrome in machado Joseph disease: Core clinical features. Cerebellum. 2012;11:549–56.
Moro A, Teive HAG. Comprometimento cognitivo na ataxia espinocerebelar do tipo 10. Dement e Neuropsychol. 2016;10:310–4.
Moro A, Munhoz RP, Moscovich M, Arruda WO, Raskin S, Silveira-Moriyama L, et al. Nonmotor Symptoms in Patients with Spinocerebellar Ataxia Type 10. Cerebellum. 2017;16:938–44.
Chirino-Pérez A, Vaca-Palomares I, Torres DL, Hernandez-Castillo CR, Diaz R, Ramirez-Garcia G, et al. Cognitive impairments in spinocerebellar ataxia type 10 and their relation to cortical thickness. Mov Disord. 2021;36(12):2910–21.
Meira AT, Arruda WO, Ono SE, Franklin GL, de Carvalho NA, Raskin S, et al. Analysis of diffusion tensor parameters in spinocerebellar ataxia type 3 and type 10 patients. Parkinsonism Relat Disord. 2020;78:73–8.
Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol - Ser Biol Sci Med Sci. 2017;72:655–61.
Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol - Ser Biol Sci Med Sci. 2009;64:896–901.
Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–35.
Ilg W, Müller B, Faber J, van Gaalen J, Hengel H, Vogt IR, et al. Digital Gait Biomarkers Allow to Capture 1-Year Longitudinal Change in Spinocerebellar Ataxia Type 3. Mov Disord. 2022;37:2295–301.
Schniepp R, Schlick C, Pradhan C, Dieterich M, Brandt T, Jahn K, et al. The interrelationship between disease severity, dynamic stability, and falls in cerebellar ataxia. J Neurol. 2016;263:1409–17.
Funding
The study does not receive any external funding
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by HC-UFPR’s Committee for Ethics in Human Research (CAAE 88366318.1.0000.0096)
Consent to Participate
All participants included in this study signed a voluntary informed consent form.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barcellos, I., Hansen, C., Strobel, G.K. et al. Spatiotemporal Gait Analysis of Patients with Spinocerebellar Ataxia Types 3 and 10 Using Inertial Measurement Units: A Comparative Study. Cerebellum (2024). https://doi.org/10.1007/s12311-024-01709-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-024-01709-7