Abstract
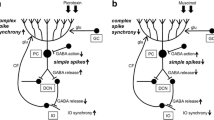
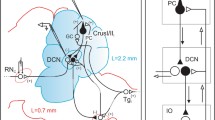
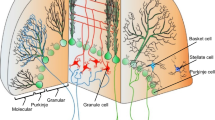
We review advances in understanding Purkinje cell (PC) complex spike (CS) physiology that suggest increased CS synchrony underlies syndromic essential tremor (ET). We searched PubMed for papers describing factors that affect CS synchrony or cerebellar circuits potentially related to tremor. Inferior olivary (IO) neurons are electrically coupled, with the degree of coupling controlled by excitatory and GABAergic inputs. Clusters of coupled IO neurons synchronize CSs within parasagittal bands via climbing fibers (Cfs). When motor cortex is stimulated in rats at varying frequencies, whisker movement occurs at ~10 Hz, correlated with synchronous CSs, indicating that the IO/CS oscillatory rhythm gates movement frequency. Intra-IO injection of the GABAA receptor antagonist picrotoxin increases CS synchrony, increases whisker movement amplitude, and induces tremor. Harmaline and 5-HT2a receptor activation also increase IO coupling and CS synchrony and induce tremor. The hotfoot17 mouse displays features found in ET brains, including cerebellar GluRδ2 deficiency and abnormal PC Cf innervation, with IO- and PC-dependent cerebellar oscillations and tremor likely due to enhanced CS synchrony. Heightened coupling within the IO oscillator leads, through its dynamic control of CS synchrony, to increased movement amplitude and, when sufficiently intense, action tremor. Increased CS synchrony secondary to aberrant Cf innervation of multiple PCs likely also underlies hotfoot17 tremor. Deep cerebellar nucleus (DCN) hypersynchrony may occur secondary to increased CS synchrony but might also occur from PC axonal terminal sprouting during partial PC loss. Through these combined mechanisms, increased CS/DCN synchrony may plausibly underlie syndromic ET.

Similar content being viewed by others
References
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87.
Handforth A, Parker GA. Conditions associated with essential tremor in veterans: a potential role for chronic stress. Tremor Other Hyperkinet Mov (N Y). 2018;8:517.
Growdon JH, Shahani BT, Young RR. The effect of alcohol on essential tremor. Neurology. 1975;25:259–62.
Hömberg V, Hefter H, Reiners K, Freund HJ. Differential effects of changes in mechanical limb properties on physiological and pathological tremor. J Neurol Neurosurg Psychiatry. 1987;50:568–79.
Pozos RS, Iaizzo PA. Effects of topical anesthesia on essential tremor. Electromyogr Clin Neurophysiol. 1992;32:369–72.
Lakie M, Walsh EG, Arblaster LA, Villagra F, Roberts RC. Limb temperature and human tremors. J Neurol Neurosurg Psychiatry. 1994;57:35–42.
Carpenter MB, Stevens GH. Structural and functional relationships between the deep cerebellar nuclei and the brachium conjunctivum in the rhesus monkey. J Comp Neurol. 1957;107:109–63.
Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol. 1977;40:1214–24.
Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. 2009;24:1629–35.
Dupuis MJ, Evrard FL, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–7.
Filip P, Lungu OV, Manto MU, Bareš M. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum. 2016;15:774–80.
Popa T, Russo M, Vidailhet M, Roze E, Lehéricy S, Bonnet C, et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimul. 2013;6:175–9.
Pedrosa DJ, Nelles C, Brown P, Volz LJ, Pelzer EA, Tittgemeyer M, et al. The differentiated networks related to essential tremor onset and its amplitude modulation after alcohol intake. Exp Neurol. 2017;297:50–61.
Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966;182:268–96.
Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Häusser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–99.
Sasaki K, Bower JM, Llinás R. Multiple Purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci. 1989;1:572–86.
Sugihara I, Lang EJ, Llinás R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol Lond. 1993;470:243–71.
Lang EJ, Sugihara I, Welsh JP, Llinás R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–39.
Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–71.
Sotelo C, Llinás R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–59.
Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–67.
Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981;315:569–84.
Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–82.
Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current Ih. J Neurophysiol. 1997;77:3145–56.
Leznik E, Makarenko V, Llinás R. Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J Neurosci. 2002;22:2804–15.
Leznik E, Llinás R. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–56.
Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–905.
Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, et al. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–8.
Belluardo N, Mudò G, Travato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, et al. Expression of connexin36 in the adult and develo** rat brain. Brain Res. 2000;865:121–38.
Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008.
Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–48.
Marshall SP, van der Giessen RS, de Zeeuw CI, Lang EJ. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum. 2007;6:287–99.
Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–75.
De Zeeuw CI, Lang EJ, Sugihara I, Ruigrok TJH, Eisenman LM, Mugnaini E, et al. Morphological correlates of bilateral synchrony in the rat cerebellar cortex. J Neurosci. 1996;16:3412–26.
Nelson BJ, Mugnaini E. Origins of GABAergic inputs to the inferior olive. In: Strata P, editor. The olivocerebellar system in motor control. Berlin: Springer-Verlag; 1989. p. 86–107.
De Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35.
Lang EJ. Organization of olivocerebellar activity in the absence of excitatory glutamatergic input. J Neurosci. 2001;21:1663–75.
Turecek J, Yuen GS, Han VZ, Zeng XH, Bayer KU, Welsh JP. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron. 2014;81:1375–88.
Sugihara I, Marshall SP, Lang EJ. Relationship of complex spike synchrony to the lobular and longitudinal aldolase C compartments in crus IIA of the cerebellar cortex. J Comp Neurol. 2007;501:13–29.
Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum. 2011;10:449–63.
Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–5.
Lang EJ, Blenkinsop TA. Control of cerebellar nuclear cells: a direct role for complex spikes? Cerebellum. 2011;10:694–701.
Tang T, Blenkinsop TA, Lang EJ. Complex spike synchrony dependent modulation of rat deep cerebellar nuclear activity. Elife. 2019;8:e40101.
Lang EJ, Tang T, Suh CY, **ao J, Kotsurovskyy Y, Blenkinsop TA, et al. Modulation of Purkinje cell complex spike waveform by synchrony levels in the olivocerebellar system. Front Syst Neurosci. 2014;8:210.
Lang EJ, Apps R, Bengtsson F, Cerminara NL, De Zeeuw CI, Ebner TJ, et al. The roles of the olivocerebellar pathway in motor learning and motor control A consensus paper. Cerebellum. 2017;16:230–52.
Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7.
Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–60.
De Gruijl JR, Hoogland TM, De Zeeuw CI. Behavioral correlates of complex spike synchrony in cerebellar microzones. J Neurosci. 2014;34:8937–47.
Tang T, Suh CY, Blenkinsop TA, Lang EJ. Synchrony is key: complex spike inhibition of the deep cerebellar nuclei. Cerebellum. 2016;15:10–3.
Lang EJ, Sugihara I, Llinás R. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rats. J Physiol Lond. 2006;571:101–20.
Horsley V, Schäfer FRS. Experiments on the character of the muscular contractions which are evoked by excitation of the various parts of the motor tract. J Physiol Lond. 1886;7:96–110.
Elble RJ. Physiologic and essential tremor. Neurology. 1986;36:225–31.
Vallbo ÅB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol. 1993;469:673–91.
Schnitzler A, Timmermann L, Gross J. Physiological and pathological oscillatory networks in the human motor system. J Physiol Paris. 2006;99:3–7.
Brown AM, White JJ, van der Heijden ME, Zhou J, Lin T, Sillitoe RV. Purkinje cell misfiring generates high-amplitude action tremors that are corrected by cerebellar deep brain stimulation. Elife. 2020;9:e51928.
De Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973;53:81–95.
Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87.
De Montigny C, Lamarre Y. Effects produced by local applications of harmaline in the inferior olive. Can J Physiol Pharmacol. 1975;53:845–9.
Beitz AJ, Saxon D. Harmaline-induced climbing fiber activation causes amino acid and peptide release in the rodent cerebellar cortex and a unique temporal pattern of Fos expression in the olivo-cerebellar pathway. J Neurocytol. 2004;33:49–74.
Llinás R, Mühlethaler M. Electrophysiology of guinea pig cerebellar nuclear cells in the in vitro brain stem–cerebellar preparation. J Physiol Lond. 1988;404:241–58.
Simantov R, Snyder SH, Oster-Granite ML. Harmaline-induced tremor in the rat: abolition by 3-acetylpyridine destruction of cerebellar climbing fibers. Brain Res. 1976;114:144–51.
Sharabi S, Daniels D, Last D, Guez D, Zivli Z, Castel D, et al. Non-thermal focused ultrasound induced reversible reduction of essential tremor in a rat model. Brain Stimul. 2019;12:1–8.
Lorden JF, Stratton SE, Mays LE, Oltmans GA. Purkinje cell activity in rats following chronic treatment with harmaline. Neuroscience. 1988;27:465–72.
Martin FC, Handforth A. Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor. Mov Disord. 2006;21:1641–9.
LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145:457–67.
Stratton SE, Lorden JF. Effect of harmaline on cells of the inferior olive in the absence of tremor: differential response of genetically dystonic and harmaline-tolerant rats. Neuroscience. 1991;41:543–9.
Lorden JF, Oltmans GA, McKeon TW, Lutes J, Beales M. Decreased cerebellar 3′,5′-cyclic guanosine monophosphate levels and insensitivity to harmaline in the genetically dystonic rat (dt). J Neurosci. 1985;5:2618–25.
Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci. 2008;28:4640–8.
Zagha E, Lang EJ, Rudy B. Kv3.3 channels at the Purkinje cell soma are necessary for generation of the classical complex spike waveform. J Neurosci. 2008;28:1291–300.
McMahon A, Fowler SC, Perney TM, Akemann W, Knöpfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci. 2004;19:3317–27.
Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol. 1995;73:2568–77.
Mignani S, Bohme GA, Birraux G, Boireau A, Jimonet P, Damour D, et al. 9-Carboxymethyl-5H,10H-imidazo[1,2-a]in deno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg Med Chem. 2002;10:1627–37.
Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80.
Shaffer CL, Hurst RS, Scialis RJ, Osgood SM, Bryce DK, Hoffmann WE, et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: the interspecies exposure-response continuum of the novel potentiator PF-4778574. J Pharmacol Exp Ther. 2013;347:212–24.
Gironell A, Pascual-Sedano B, Marín-Lahoz J. Perampanel, a new hope for essential tremor: an open label trial. Parkinsonism Relat Disord. 2019;60:171–2.
Handforth A, Tse W, Elble RJ. A pilot double-blind randomized trial of perampanel for essential tremor. Mov Disord Clin Pract. 2020;7:399–404.
Tomiyama M, Palacios JM, Cortés R, Mengod G. Flip and flop variants of AMPA receptor subunits in the human cerebellum: implication for the selective vulnerability of Purkinje cells. Synapse. 1999;31:163–7.
Masugi-Tokita M, Tarusawa E, Watanabe M, Molnár E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–44.
Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, et al. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology. 2010;59:380–7.
Park YG, Park HY, Lee CJ, Choi S, Jo S, Choi H, et al. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci U S A. 2010;107:10731–6.
Ondo WG. Current and emerging treatments of essential tremor. Neurol Clin. 2020;38:309–23.
Handforth A. Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov (N Y). 2012;2:02–92–769-1.
Sinton CM, Krosser BI, Walton KD, Llinás RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflugers Arch. 1989;414:31–6.
Nahab FB, Wittevrongel L, Ippolito D, Toro C, Grimes GJ, Starling J, et al. An open-label, single-dose, crossover study of the pharmacokinetics and metabolism of two oral formulations of 1-octanol in patients with essential tremor. Neurotherapeutics. 2011;8:753–62.
Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. 1996;39:650–8.
Batini C, Buisseret-Delmas C, Conrath-Verrier M. Harmaline-induced tremor. I. Regional metabolic activity as revealed by [14C]2-deoxyglucose in cat. Exp Brain Res. 1981;42:371–82.
Miwa H, Nishi K, Fuwa T, Mizuno Y. Differential expression of c-fos following administration of two tremorgenic agents: harmaline and oxotremorine. Neuroreport. 2000;11:2385–90.
Tian JB, Bishop GA. Stimulus-dependent activation of c-Fos in neurons and glia in the rat cerebellum. J Chem Neuroanat. 2002;23:157–70.
Kosmowska B, Ossowska K, Głowacka U, Wardas J. Tremorolytic effect of 5′-chloro-5′-deoxy-(±)-ENBA, a potent and selective adenosine A1 receptor agonist, evaluated in the harmaline-induced model in rats. CNS Neurosci Ther. 2017;23:438–46.
Kosmowska B, Ossowska K, Konieczny J, Lenda T, Berghauzen-Maciejewska K, Wardas J. Inhibition of excessive glutamatergic transmission in the ventral thalamic nuclei by a selective adenosine A1 receptor agonist, 5′-chloro-5′-deoxy-(±)-ENBA underlies its tremorolytic effect in the harmaline-induced model of essential tremor. Neuroscience. 2020;429:106–18.
Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80.
Lee J, Chang SY. Altered primary motor cortex neuronal activity in a rat model of harmaline-induced tremor during thalamic deep brain stimulation. Front Cell Neurosci. 2019;13:448.
Pahapill PA, Levy R, Dostrovsky JO, Davis KD, Rezai AR, Tasker RR, et al. Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Ann Neurol. 1999;46:249–52.
Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–34.
Wiklund L, Sjölund B, Björklund A. Morphological and functional studies on the serotoninergic innervation of the inferior olive. J Physiol Paris. 1981;77:183–6.
Barragan LA, Delhaye-Bouchaud N, Laget P. Drug-induced activation of the inferior olivary nucleus in young rabbits. Differential effects of harmaline and quipazine. Neuropharmacology. 1985;24:645–54.
Costall B, Kelly DM, Naylor RJ. The importance of 5-hydroxytryptamine for the induction of harmine tremor and its antagonism by dopaminergic agonists assessed by lesions of the midbrain raphe nuclei. Eur J Pharmacol. 1976;35:109–19.
Mehta H, Saravanan KS, Mohanakumar KP. Serotonin synthesis inhibition in olivo-cerebellar system attenuates harmaline-induced tremor in Swiss albino mice. Behav Brain Res. 2003;145:31–6.
Arshaduddin M, Kadasah S, Al Deeb S, Al Moutaery K, Tariq M. Exacerbation of harmaline-induced tremor by imipramine. Pharmacol Biochem Behav. 2005;81:9–14.
Arshaduddin M, Al Kadasah S, Biary N, Al Deeb S, Al Moutaery K, Tariq M. Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res. 2004;153:15–20.
Lamarre Y, Mercier LA. Neurophysiological studies of harmaline-induced tremor in the cat. Can J Physiol Pharmacol. 1971;49:1049–58.
Alviña K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–8.
Hoebeek FE, Witter L, Ruigrok TJ, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci U S A. 2010;107:8410–5.
Bengtsson F, Ekerot CF, Jörntell H. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PLoS One. 2011;6:e18822.
Dykstra S, Engbers JD, Bartoletti TM, Turner RW. Determinants of rebound burst responses in rat cerebellar nuclear neurons to physiological stimuli. J Physiol. 2016;594:985–1003.
Barbagallo G, Arabia G, Novellino F, Nisticò R, Salsone M, Morelli M, et al. Increased glutamate + glutamine levels in the thalamus of patients with essential tremor: a preliminary proton MR spectroscopic study. Parkinsonism Relat Disord. 2018;47:57–63.
Kobayashi K, Katayama Y, Kasai M, Oshima H, Fukaya C, Yamamoto T. Localization of thalamic cells with tremor-frequency activity in Parkinson’s disease and essential tremor. Acta Neurochir Suppl. 2003;87:137–9.
Hanson TL, Fuller AM, Lebedev MA, Turner DA, Nicolelis MA. Subcortical neuronal ensembles: an analysis of motor task association, tremor, oscillations, and synchrony in human patients. J Neurosci. 2012;32:8620–32.
Najac M, Raman IM. Integration of Purkinje cell inhibition by cerebellar nucleo-olivary neurons. J Neurosci. 2015;35:544–9.
Uusisaari M, Obata K, Knopfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–11.
Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–62.
Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34:82–90.
Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–42.
Conti V, Aghaie A, Cilli M, Martin N, Caridi G, Musante L, et al. crv4, a mouse model for human ataxia associated with kyphoscoliosis caused by an mRNA splicing mutation of the metabotropic glutamate receptor 1 (Grm1). Int J Mol Med. 2006;18:593–600.
Rossi PI, Musante I, Summa M, Pittaluga A, Emionite L, Ikehata M, et al. Compensatory molecular and functional mechanisms in nervous system of the Grm1crv4 mouse lacking the mGlu1 receptor: a model for motor coordination deficits. Cereb Cortex. 2013;23:2179–89.
Kolasiewicz W, Kuter K, Wardas J, Ossowska K. Role of the metabotropic glutamate receptor subtype 1 in the harmaline-induced tremor in rats. J Neural Transm. 2009;116:1059–63.
Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342:21–7.
Chuang WL, Huang YZ, Lu CS, Chen RS. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov Disord. 2014;29:501–7.
Sinclair JG, Lo GF, Harris DP. Ethanol effects on the olivocerebellar system. Can J Physiol Pharmacol. 1982;60:610–4.
Rappaport MS, Gentry RT, Schneider DR, Dole VP. Ethanol effects on harmaline-induced tremor and increase of cerebellar cyclic GMP. Life Sci. 1984;34:49–56.
Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305.
Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type a receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–23.
Kato K. Novel GABAA, receptor α subunit is expressed only in cerebellar granule cells. J Mol Biol. 1990;214:619–24.
Hortnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–72.
Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–76.
Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–62.
Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216.
Handforth A, Kadam PA, Kosoyan HP, Eslami P. Suppression of harmaline tremor by activation of an extrasynaptic GABAA receptor: implications for essential tremor. Tremor Other Hyperkinet Mov (N Y). 2018;8:546.
Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106:2057–64.
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre–Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–59.
Lee D, Gan SR, Faust PL, Louis ED, Kuo SH. Climbing fiber–Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord. 2018;51:24–9.
Watanabe M. Molecularmechanisms governing competitive synaptic wiring in cerebellar Purkinje cells. Tohoku J Exp Med. 2008;214:175–90.
Mishina M, Uemura T, Yasumura M, Yoshida T. Molecular mechanism of parallel fiber–Purkinje cell synapse formation. Front Neural Circuits. 2012;6:90.
Miyazaki T, Yamasaki M, Takeuchi T, Sakimura K, Mishina M, Watanabe M. Ablation of glutamate receptor GluRδ2 in adult Purkinje cells causes multiple innervation of Cfs by inducing aberrant invasion to parallel fiber innervation territory. J Neurosci. 2010;30:15196–209.
Pan MK, Li YS, Wong SB, Ni CL, Wang YM, Liu WC, et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med. 2020;12(526):eaay1769.
Hashizume M, Miyazaki T, Sakimura K, Watanabe M, Kitamura K, Kano M. Disruption of cerebellar microzonal organization in GluD2 (GluRδ2) knockout mouse. Front Neural Circuits. 2013;7:130.
Good JM, Mahoney M, Miyazaki T, Tanaka KF, Sakimura K, Watanabe M, et al. Maturation of cerebellar Purkinje cell population activity during postnatal refinement of climbing fiber network. Cell Rep. 2017;21:2066–73.
Louis ED, Babij R, Lee M, Cortés E, Vonsattel JP. Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–9.
Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61.
Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res. 2007;1140:51–74.
Grüsser-Cornehls U, Grüsser C, Bäurle J. Vermectomy enhances parvalbumin expression and improves motor performance in Weaver mutant mice: an animal model for cerebellar ataxia. Neuroscience. 1999;91:315–26.
Roffler-Tarlov S, Beart PM, O'Gorman S, Sidman RL. Neurochemical and morphological consequences of axon terminal degeneration in cerebellar deep nuclei of mice with inherited Purkinje cell degeneration. Brain Res. 1979;168:75–95.
Bäurle J, Grover BG, Grüsser-Cornehls U. Plasticity of GABAergic terminals in Deiters’ nucleus of weaver mutant and normal mice: a quantitative light microscopic study. Brain Res. 1992;591:305–18.
Roffler-Tarlov S, Turey M. The content of amino acids in the develo** cerebellar cortex and deep cerebellar nuclei of granule cell deficient mutant mice. Brain Res. 1982;247:65–73.
Handforth A. Linking essential tremor to the cerebellum-animal model evidence. Cerebellum. 2016;15:285–98.
Louis ED, Lee M, Cortés E, Vonsattel JP, Faust PL. Matching asymmetry of tremor with asymmetry of postmortem cerebellar hemispheric changes in essential tremor. Cerebellum. 2014;13:462–70.
Louis ED, Hernandez N, Dyke JP, Ma RE, Dydak U. In vivo dentate nucleus gamma-aminobutyric acid concentration in essential tremor vs. controls. Cerebellum. 2018;17:165–72.
Nandy K. Morphological changes in the cerebellar cortex of aging Macaca nemestrina. Neurobiol Aging. 1981;2:61–4.
Sturrock RR. Age related changes in Purkinje cell number in the cerebellar nodulus of the mouse. J Hirnforsch. 1989;30:757–60.
Lolova I, Davidoff M. Immuno- and histochemical data on changed GABA transmission in aged rat cerebellum. J Hirnforsch. 1990;31:423–8.
Louis ED, Diaz DT, Kuo SH, Gan SR, Cortes EP, Vonsattel JPG, et al. Inferior olivary nucleus degeneration does not lessen tremor in essential tremor. Cerebellum Ataxias. 2018;5:1.
Elkouzi A, Kattah JC, Elble RJ. Hypertrophic olivary degeneration does not reduce essential tremor. Mov Disord Clin Pract. 2015;3:209–11.
Verhaart WJ, Voogd J. Hypertrophy of the inferior olives in the cat. J Neuropathol Exp Neurol. 1962;21:92–104.
Louis ED, Lenka A. The olivary hypothesis of essential tremor: time to lay this model to rest? Tremor Other Hyperkinet Mov (N Y). 2017;7:473.
McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985;359:120–30.
Kronenbuerger M, Tronnier VM, Gerwig M, Fromm C, Coenen VA, Reinacher P, et al. Thalamic deep brain stimulation improves eyeblink conditioning deficits in essential tremor. Exp Neurol. 2008;211:387–96.
Acknowledgments
We thank Hovsep Kosoyan, Ph.D. for assistance in figure preparation.
Funding
Supported by International Essential Tremor Foundation and by Veterans Affairs (A.H.), and by a NIH/NINDS grant R21NS101386 (E.J.L.).
Author information
Authors and Affiliations
Contributions
The first draft and literature search were performed by A.H. Both E.J.L. and A.H. contributed conceptually and to critical revisions of the work.
Corresponding author
Ethics declarations
Conflict of Interest
A.H. has received funding for clinical trials from Eisai, Medtronic, and Sonexa, and is on the Medical Board of the International Essential Tremor Foundation. E.J.L. declares no conflicts of interest.
Ethics Statement
Statement of Human and Animal Rights: This article does not contain any original studies with humans or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Handforth, A., Lang, E.J. Increased Purkinje Cell Complex Spike and Deep Cerebellar Nucleus Synchrony as a Potential Basis for Syndromic Essential Tremor. A Review and Synthesis of the Literature. Cerebellum 20, 266–281 (2021). https://doi.org/10.1007/s12311-020-01197-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01197-5