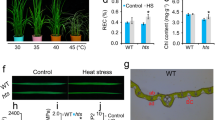
Abstract
Heliotropium thermophilum can survive at a soil temperature of 65 °C in natural and laboratory conditions, but the mechanism of survival at high soil temperatures is not completely known. The objective of this study was to determine whether changes in abscisic acid (ABA), osmolytes and heat shock factors (HSFs) levels have an effective role in the development of thermotolerance in H. thermophilum at high temperatures. Soil temperature at which the thermophilic plant could live was gradually increased in laboratory conditions and the effects of four different temperatures (20 ± 5 °C: low, 40 ± 5 °C: mild, 60 ± 5 °C: medium, 80 ± 5 °C: extreme heat) were observed for 15 days. The results showed that the content of thiobarbituric acid reactive substances (TBARS) did not significantly change in extreme heat, whereas the leaf water potential and stomatal conductivity decreased. ABA biosynthesis, accumulation of osmolyte compounds including proline and total soluble sugars, and the expression levels of heat shock transcription factor A4A (HSFA4A), heat shock transcription factor A3 (HSFA3), and heat shock factor (HSF4) genes significantly increased with increase of soil temperature from 20 ± 5 °C to 80 ± 5 °C. In conclusion, we observed that H. thermophilum is an extreme thermophile. This plant can adjust osmotic activity to effectively take water through the osmolytes accumulation, reducing water loss by ABA-mediated stomatal closing and survive at high soil temperatures by stimulating the increased transcription level of HSFs.





Similar content being viewed by others
References
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Campbell JL, Klueva NY, Zheng G, Nieto SJ, Ho THD, Nguyen HT (2001) Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochim Biophys Acta 1517:270–277
Ciura J, Kruk J (2018) Phytohormones as targets for improving plant productivity and stress tolerance. J Plant Physiol 229:32–40
Cohen Y, Moreshet S, Fuchs M (1987) Changes in hydraulic conductance of citrus trees following a reduction in wetted soil volume. Plant Cell Environ 10:53–57
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fitter AH, Hay RKM (2002) Environmental Physiology of Plants, 3rd edn. Academic Press, London, UK
Farooq M, Basra S, Wahid A, Cheema Z, Cheema M, Khaliq A (2008) Physiological role of exogenously applied glycine betaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J Agro Crop Sci 194:325–333
Gür A, Demirel U, Özden M, Kahraman A, Çopur O (2010) Diurnal gradual heat stress affects antioxidant enzymes, proline accumulation and some physiological components in cotton (Gossypium hirsutum L.). Afr J JBiotechnol 9:1008–1015
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. kinetics and stoichiometry of fatty acid peroxidation. Arch Bio Biophys 125:189–198
Hsieh EJ, Cheng MC, Lin TP (2013) Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol Biol 82:223–237
Iba K (2002) Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245
Islam MR, Baohua F, Tingting C, Guanfu F (2018) Role of Abscisic acid in thermal acclimation of plants. J Plant Biol 61:255–264
Lange PJ (2014) A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex. Phytokeys 40:185p
Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146:748–761
Li H, Liu SS, Yi CY, Wang F, Zhou J, **a XJ, Shi K, Zhou YH, Yu JQ (2014) Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ 37:2768–2780
Li Z-G, Yang SZ, Long WB, Yang GX, Shen ZZ (2013) Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 36:1564–1572
Liu HC, Charng YY (2013) Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol 163:276–290
Meng G, **-Hong L, **ao M, De-Xu L, Zhen-Hui G, Ming-Hui Lu (2016) The plant heat stress transcription factors (hsfs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci 7:114
Osakabe Y, Osakabe K, Shinozaki K, Tran LP (2014) Response of plants to water stress. Front Plant Sci 5:86–94
Oztürk K, Sağlam A, Kadioğlu A (2020) Heliotropium thermophilum, an extreme heat tolerant species, promises plants about adaptation to high soil temperature conditions. Physiol Mol Bio Plants 26:525–535
Perez-Rodriguez P, Riano-Pachon DM, Correa LG, Rensing SA, Kersten B, Mueller-Roeber B (2010) PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res 38:822–827
Rossi S, Burgess P, Jespersen D, Huang B (2017) Heat-induced leaf senescence associated with chlorophyll metabolism in bentgrass lines differing in heat tolerance. Crop Sci 57:169–178
Sakamoto A, Murata N (2000) genetic engineering of glycine betaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51:81–88
Sairam R, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Savage MJ, Cass A (1984) Psychrometric field measurement of water potential changes following leaf excision. Plant Physiol 74:96–98
Savicka M, Škute N (2010) Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 56:26–33
Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochem Biophys Acta 1819:104–119
Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, Von Koskull-Döring P (2008) A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J 53:264–274
Smale M, Fitzgerald N (2015) A field guide to the vegetation associations of the Taupo Volcanic Zone, Landcare Research New Zealand, Waikato Regional Council Technical Report, 32, ISSN 2230-4355.
Tan K, Çelik A, Gemici Y, Gemici M, Yildirim H (2008) Heliotropium thermophilum (Boraginaceae), a new taxon from SW Anatolia. Turkey Adv Sci Lett 1:132–139
Tercek MT, Hauber DP, Darwin SP (2003) Genetic and historical relationships among geothermally adapted Agrostis (bentgrass) North America and Kamchatka: evidence for apreviously unrecognized thermally adapted taxon. Am J Bot 90:1306–1312
Wahid A (2007) Physiological implications of metabolites biosynthesis in net assimilation and heat stress tolerance of sugarcane (Saccharum officinarum) Sprouts. J Plant Res 120:219–228
Wang X, Zhuang L, Shi Y, Huang B (2017) Up-Regulation of HSFA2c and HSPs by ABA contributing to improved heat tolerance in tall fescue and Arabidopsis. Int J Mol Sci 18:1981
Xu Y, Du H, Huang B (2013) Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling. Crop Sci 53:1626–1635
Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D et al (2011) Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shockresponsive gene expression. Mol Genet Genomics 286:321–332
Acknowledgements
This study was supported by the project at Karadeniz Technical University Research Projects Unit (Project No: FDK-2017-7214). We also thank to native speaker Russell Fraser for English language improvement.
Author information
Authors and Affiliations
Contributions
ASM and AK designed the research. ASM performed the experiments. ASM and AK analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sezgin Muslu, A., Kadıoğlu, A. Role of abscisic acid, osmolytes and heat shock factors in high temperature thermotolerance of Heliotropium thermophilum. Physiol Mol Biol Plants 27, 861–871 (2021). https://doi.org/10.1007/s12298-021-00975-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00975-7