Abstract
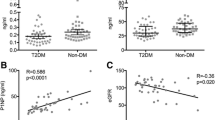
The study evaluates Asprosin's value in diabetic postmenopausal women, examining its reliability as a predictor for osteoporosis (OP) in the second type of diabetes (T2D) women. A case–control study recruited 255 postmenopausal women attending the geriatric department of the University Hospital. They were grouped into controls (non-OP non-T2D), and study cases. The latter were subdivided into: non-OP T2D, and OP T2D postmenopausal women (85/255) for each. Serum Asprosin level showed a significant increase in postmenopausal T2D women with OP (42.51 ± 2.97 ng/mL, P < 0.001) compared with postmenopausal T2D women without OP and controls. Additionally, there is a significant interrelationship between OP radiological indicators and bone-forming hormone in T2D women, osteocalcin. Moreover, bone resorption and glycemic markers in T2D women correlated significantly and positively with Asprosin. The Receiver operator characteristic curve discriminates OP T2D postmenopausal women from non-OP T2D postmenopausal women by estimating cutoff value (> 39.3 ng/mL) at 90% sensitivity, 63.3% specificity, and P < 0.001.


Similar content being viewed by others
Abbreviations
- OP:
-
Osteoporosis
- DXA:
-
Dual-energy x-ray absorptiometry
- BMD:
-
Bone mineral density
- WAT:
-
White adipose tissue
- IR:
-
Insulin resistance
- T2D:
-
Type 2 diabetes
- AGEs:
-
The advanced glycation end products
- BMI:
-
Body mass index
- HbA1c:
-
Glycated hemoglobin
- OC:
-
Serum (Osteocalcin)
- PTH:
-
Parathyroid hormone
- CTX:
-
Serum human C-terminal telopeptides of types I collagen
- MDA:
-
Serum malondialdehyde
- SOD:
-
Superoxide dismutase
- ALP:
-
Serum bone alkaline phosphatase
- HOMA-IR:
-
Hemostatic model assessment-insulin resistance
- ROC:
-
The receiver operator characteristic curve
References
Bover J, Bailone L, López-Báez V, Benito S, Ciceri P, Galassi A, et al. Osteoporosis, bone mineral density and CKD–MBD: treatment considerations. J Nephrol. 2017;30:677–87.
Marinho BCG, Guerra LP, Drummond JB, Silva BC, Soares MMS. The burden of osteoporosis in Brazil. Arquivos Brasileiros de Endocrinol Metabol. 2014;58:434–43.
Merle B, Haesebaert J, Bedouet A, Barraud L, Flori M, Schott A-M, et al. Osteoporosis prevention: Where are the barriers to improvement in French general practitioners? A qualitative study. PLoS ONE. 2019;14(7): e0219681.
Mariani G, Kasznia-Brown J, Paez D, Mikhail MN, Salama DH, Bhatla N, et al. Improving women’s health in low-income and middle-income countries. Part I: challenges and priorities. Nucl Med Commun. 2017;38(12):1019.
Hassan WN, Shallal F, Roomi AB. Prediction of successful Induction of labor using ultrasonic fetal parameters. Current Women’s Health Rev. 2022;18(1):134–9.
Radecka A, Lubkowska A. The significance of dual-energy x-ray absorptiometry (DXA) examination in cushing’s syndrome—A systematic review. Diagnostics. 2023;13(9):1576.
Kuo T-R, Chen C-H. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomarker Res. 2017;5(1):1–9.
Marini S, Barone G, Masini A, Dallolio L, Bragonzoni L, Longobucco Y, et al. The effect of physical activity on bone biomarkers in people with osteoporosis: a systematic review. Front Endocrinol. 2020;11: 585689.
Farrag M, Ait Eldjoudi D, González-Rodríguez M, Cordero-Barreal A, Ruiz-Fernández C, Capuozzo M, et al. Asprosin in health and disease, a new glucose sensor with central and peripheral metabolic effects. Front Endocrinol. 2023;13:1101091.
Hekim MG, Kelestemur MM, Bulmus FG, Bilgin B, Bulut F, Gokdere E, et al. Asprosin, a novel glucogenic adipokine: a potential therapeutic implication in diabetes mellitus. Arch Physiol Biochem. 2021;129:1–7.
Cavati G, Pirrotta F, Merlotti D, Ceccarelli E, Calabrese M, Gennari L, et al. Role of advanced glycation end-products and oxidative stress in type-2-diabetes-induced bone fragility and implications on fracture risk stratification. Antioxidants. 2023;12(4):928.
Zhang L, Chen C, Zhou N, Fu Y, Cheng X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta. 2019;489:183–8.
Roomi AB, Nori W, Al-Badry SH. The value of serum adiponectin in osteoporotic women: does weight have an effect? J Obesity. 2021;2021.
Al-Jawadi WA, Jankeer MH. Study of the balance system between some enzymatic and non-enzymatic antioxidants in blood serum of patients with rheumatoid arthritis in Mosul City, Iraq. Medico-Legal Update. 2021;21(1).
Fox C, Bernardino L, Cochran J, Essig M, Bridges KG. Inappropriate use of homeostasis model assessment cutoff values for diagnosing insulin resistance in pediatric studies. J Am Osteopath Assoc. 2017;117(11):689–96.
Czerwiński E, Badurski JE, Marcinowska-Suchowierska E, Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil. 2007;9(4):337–56.
Chow S-C, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research: CRC press; 2017.
Clemente-Suárez VJ, Redondo-Flórez L, Beltrán-Velasco AI, Martín-Rodríguez A, Martínez-Guardado I, Navarro-Jiménez E, et al. The role of adipokines in health and disease. Biomedicines. 2023;11(5):1290.
Morcos YA, Lütke S, Tenbieg A, Hanisch F-G, Pryymachuk G, Piekarek N, et al. Sensitive asprosin detection in clinical samples reveals serum/saliva correlation and indicates cartilage as source for serum asprosin. Sci Rep. 2022;12(1):1340.
Naiemian S, Naeemipour M, Zarei M, Lari Najafi M, Gohari A, Behroozikhah MR, et al. Serum concentration of asprosin in new-onset type 2 diabetes. Diabetol Metab Syndr. 2020;12:1–8.
Groener JB, Valkanou A, Kender Z, Pfeiffenberger J, Kihm L, Fleming T, et al. Asprosin response in hypoglycemia is not related to hypoglycemia unawareness but rather to insulin resistance in type 1 diabetes. PLoS ONE. 2019;14(9): e0222771.
Li X, Liao M, Shen R, Zhang L, Hu H, Wu J, et al. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediat Inflammat. 2018;2018.
Wang C-Y, Lin T-A, Liu K-H, Liao C-H, Liu Y-Y, Wu VC-C, et al. Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes. 2019;43(5):1019–25.
Wang Y, Qu H, **ong X, Qiu Y, Liao Y, Chen Y, et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediat Inflamm. 2018;2018.
Cantay H, Binnetoglu K, Gul HF, Bingol SA. Investigation of serum and adipose tissue levels of asprosin in patients with severe obesity undergoing sleeve gastrectomy. Obesity. 2022;30(8):1639–46.
Gozel N, Kilinc F. Investigation of plasma asprosin and saliva levels in newly diagnosed type 2 diabetes mellitus patients treated with metformin. Endokrynol Pol. 2021;72(1):37–43.
Kantorowicz M, Szymura J, Szygula Z, Kusmierczyk J, Maciejczyk M, Wiecek M. Nordic walking at maximal fat oxidation intensity decreases circulating asprosin and visceral obesity in women with metabolic disorders. Front Physiol. 2021;12: 726783.
Zhang Y, Huang X, Sun K, Li M, Wang X, Han T, et al. The potential role of serum IGF-1 and leptin as biomarkers: towards screening for and diagnosing postmenopausal osteoporosis. J Inflamm Res. 2022:533–43.
Mohsin S, Baniyas MM, AlDarmaki RS, Tekes K, Kalász H, Adeghate EA. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin Biol Ther. 2019;19(9):937–48.
Yuan M, Li W, Zhu Y, Yu B, Wu J. Asprosin: a novel player in metabolic diseases. Front Endocrinol. 2020;11:64.
Haie, CHEN Hongyan, CHEN Hongjiao. The correlation between serum Asprosin levels and bone mineral density, balance ability fracture incidence in postmenopausal women, Chin J Osteopros, Feb 2021, 27:2.
Alobaidi MBA, Al-Samarrai RRH. Correlation between serum asprosin level and oxidative stress in Iraqi patients with type II diabetes mellitus. Sys Rev Pharm. 2020;11(12):1729–33.
Ali EA, Tahseen YH, El-Yassin HD. Thyroid Disorders and the Level of Malondialdehyde. J Fac Med Baghdad. 2009;51(1):67–70.
Bover J, Ureña-Torres P, Torregrosa J-V, Rodríguez-García M, Castro-Alonso C, Górriz JL, et al. Osteoporosis, bone mineral density and CKD–MBD complex (I): diagnostic considerations. Nefrología (English Edition). 2018;38(5):476–90.
Mishra I, Duerrschmid C, Ku Z, He Y, **e W, Silva ES, et al. Asprosin-neutralizing antibodies as a treatment for metabolic syndrome. Elife. 2021;10: e63784.
Shi X, Jiang J, Hong R, Xu F, Dai S. Circulating IGFBP-3 and interleukin 6 as predictors of osteoporosis in Postmenopausal Women: a cross-sectional study. Mediat Inflamm. 2023;2023.
Seyhanli ES, Koyuncu I, Yasak IH, Demir HA, Temiz E. Asprosin and oxidative stress level in COVID-19 patients. Clin Lab. 2022;68(1).
Nori W, Zghair MAG. Omicron targets upper airways in pediatrics, elderly and unvaccinated population. World J Clin Cases. 2022;10(32):12062.
Ali AI, Nori W. Correlation of serum visfatin level in non-obese women with polycystic ovary syndrome and matched control. Reprod Sci. 2022;29(11):3285–93.
Acknowledgements
The authors wish to thank the Ministry of Higher Education and Scientific Research in Iraq.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have disclosed any conflicts of interest that would have been perceived as having influenced the work presented in this paper, either financially or otherwise.
Ethical Approval
The information used in this study was obtained with approval from the Iraqi Ministry of Health. Sampling was Parental permission using informed consent. Participants' personal data is encrypted to ensure anonymity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roomi, A.B., Ali, E.A., Nori, W. et al. Asprosin is a Reliable Predictor of Osteoporosis in Type 2 Diabetic Postmenopausal Women: A Case–Control Study. Ind J Clin Biochem (2023). https://doi.org/10.1007/s12291-023-01163-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-023-01163-y