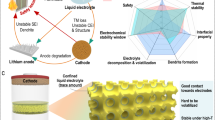
Abstract
Electrolytes with high-efficiency lithium-ion transfer and reliable safety are of great importance for lithium battery. Although having superior ionic conductivity (10−3–10−2 S·cm−1), traditional liquid-state electrolytes always suffer from low lithium-ion transference number \(({t_{{\rm{L}}{{\rm{i}}^ + }}},\,\, < 0.4)\) and thus undesirable battery performances. Herein, the deep eutectic solvent (DES) is vacuum-filtered into the ∼ 1 nm interlayer channel of vermiculite (Vr) lamellar framework to fabricate a quasi-solid electrolyte (Vr-DES QSE). We demonstrate that the nanoconfinement effect of interlayer channel could facilitate the opening of solvation shell around lithium-ion. Meanwhile, the interaction from channel wall could inhibit the movement of anion. These enable high-efficiency lithium-ion transfer: 2.61 × 10−4 S·cm−1 at 25 °C. Importantly, the \({t_{{\rm{L}}{{\rm{i}}^ + }}}\) value reaches 0.63, which is 4.5 times of that of bulk DES, and much higher than most present liquid/quasi-solid electrolytes. In addition, Vr-DES QSE shows significantly improved interfacial stability with Li anode as compared with DES. The assembled Li symmetric cell can operate stably for 1000 h at 0.1 mA·cm−2. The lithium iron phosphate (LFP)∣Vr-DES QSE∣Li cell exhibits high capacity of 142.1 mAh·g−1 after 200 cycles at 25 °C and 0.5 C, with a capacity retention of 94.5%. The strategy of open solvation shell through nanoconfinement effect of lamellar framework may shed light on the development of advanced electrolytes.

Similar content being viewed by others
References
Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456.
Goodenough, J. B. How we made the Li-ion rechargeable battery. Nat. Electron. 2018, 1, 204.
Li, J. J.; Hu, H. M.; Fang, W. H.; Ding, J. W.; Yuan, D.; Luo, S. J.; Zhang, H. T.; Ji, X. Y. Impact of fluorine-based lithium salts on SEI for all-solid-state PEO-based lithium metal batteries. Adv. Funct. Mater. 2023, 33, 2303718.
Yao, M.; Ruan, Q. Q.; Pan, S. S.; Zhang, H. T.; Zhang, S. J. An ultrathin asymmetric solid polymer electrolyte with intensified ion transport regulated by biomimetic channels enabling wide-temperature high-voltage lithium-metal battery. Adv. Energy Mater. 2023, 13, 2203640.
Mishra, K.; Devi, N.; Siwal, S. S.; Zhang, Q. B.; Alsanie, W. F.; Scarpa, F.; Thakur, V. K. Ionic liquid-based polymer nanocomposites for sensors, energy, biomedicine, and environmental applications: Roadmap to the future. Adv. Sci. 2022, 9, 2202187.
Jeoun, Y.; Kim, K.; Kim, S. Y.; Lee, S. H.; Huh, S. H.; Kim, S. H.; Huang, X.; Sung, Y. E.; Abruña, H. D.; Yu, S. H. Surface roughness-independent homogeneous lithium plating in synergetic conditioned electrolyte. ACS Energy Lett. 2022, 7, 2219–2227.
Amanchukwu, C. V.; Yu, Z. A.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability. J. Am. Chem. Soc. 2020, 142, 7393–7403.
Ruan, Q. Q.; Yao, M.; Yuan, D.; Dong, H. T.; Liu, J. X.; Yuan, X. D.; Fang, W. H.; Zhao, G. Y.; Zhang, H. T. Ionic liquid crystal electrolytes: Fundamental, applications and prospects. Nano Energy 2023, 106, 108087.
Giffin, G. A. The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries. Nat. Commun. 2022, 13, 5250.
Yu, Z.; Balsara, N. P.; Borodin, O.; Gewirth, A. A.; Hahn, N. T.; Maginn, E. J.; Persson, K. A.; Srinivasan, V.; Toney, M. F.; Xu, K. et al. Beyond local solvation structure: Nanometric aggregates in battery electrolytes and their effect on electrolyte properties. ACS Energy Lett. 2022, 7, 461–470.
Zhou, P.; Zhang, X. K.; **ang, Y.; Liu, K. Strategies to enhance Li+ transference number in liquid electrolytes for better lithium batteries. Nano Res. 2023, 16, 8055–8071.
Qiao, B.; Leverick, G. M.; Zhao, W.; Flood, A. H.; Johnson, J. A.; Shao-Horn, Y. Supramolecular regulation of anions enhances conductivity and transference number of lithium in liquid electrolytes. J. Am. Chem. Soc. 2018, 140, 10932–10936.
Nan, B.; Chen, L.; Rodrigo, N. D.; Borodin, O.; Piao, N.; **a, J. L.; Pollard, T.; Hou, S.; Zhang, J. X.; Ji, X. et al. Enhancing Li+ transport in NMC811 graphite lithium-ion batteries at low temperatures by using low-polarity-solvent electrolytes. Angew. Chem., Int. Ed. 2022, 61, e202205967.
Xu, Y.; Yan, H. H.; Li, T.; Liu, Y.; Luo, J. M.; Li, W. Y.; Cui, X. Y.; Chen, L.; Yue, Q.; Kang, Y. J. Can carbon sponge be used as separator in Li metal batteries. Energy Storage Mater. 2021, 36, 108–114.
Jiang, Y. X.; Song, Y. D.; Chen, X.; Wang, H. J.; Deng, L. J.; Yang, G. In situ formed self-healable quasi-solid hybrid electrolyte network coupled with eutectic mixture towards ultra-long cycle life lithium metal batteries. Energy Storage Mater. 2022, 52, 514–523
Wang, Y. J.; Li, L. B.; Wei, Y. Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H. H. Water transport with ultralow friction through partially exfoliated g-C3N4 nanosheet membranes with self-supporting spacers. Angew. Chem., Int. Ed. 2017, 56, 8974–8980.
Hu, C. Y.; Achari, A.; Rowe, P.; **ao, H.; Suran, S.; Li, Z.; Huang, K.; Chi, C.; Cherian, C. T.; Sreepal, V. et al. pH-de pendent water permeability switching and its memory in MoS2 membranes. Nature 2023, 616, 719–723
Jun, B. M.; Kim, S.; Heo, J.; Park, C. M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2019, 12, 471–487.
Wang, J.; Zhou, H. J.; Li, S. Z.; Wang, L. Selective ion transport in two-dimensional lamellar nanochannel membranes. Angew. Chem., Int. Ed. 2023, 62, e202218321.
Ding, L.; Wei, Y. Y.; Li, L. B.; Zhang, T.; Wang, H. H.; Xue, J.; Ding, L. X.; Wang, S. Q.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155.
Jia, Z. M.; Li, X. F.; Zhang, J.; He, N. N.; Long, H. H.; Zou, Y. D.; Zhang, Y. D.; Jiang, B.; Qi, Y.; Li, Y. et al. Monodisperse covalent organic nanosheets by in-situ oxidation method for efficient ion/molecule separation. J. Membr. Sci. 2023, 683, 121783.
Jia, W.; Wu, B. H.; Sun, S. T.; Wu, P. Y. Interfacially stable MOF nanosheet membrane with tailored nanochannels for ultrafast and thermo-responsive nanofiltration. Nano Res. 2020, 13, 2973–2978.
Sapkota, B.; Liang, W. T.; VahidMohammadi, A.; Karnik, R.; Noy, A.; Wanunu, M. High permeability sub-nanometre sieve composite MoS2 membranes. Nat. Commun. 2020, 11, 2747.
Zhang, Y. F.; Huang, J. J.; Liu, H.; Kou, W. J.; Dai, Y.; Dang, W.; Wu, W. J.; Wang, J. T.; Fu, Y. Z.; Jiang, Z. Y. Lamellar ionic liquid composite electrolyte for wide-temperature solid-state lithium-metal battery. Adv. Energy Mater. 2023, 13, 2300156.
Wu, J. X.; Liang, Q. H.; Yu, X. L.; Lü, Q. F.; Ma, L. B.; Qin, X. Y.; Chen, G. H.; Li, B. H. Deep eutectic solvents for boosting electrochemical energy storage and conversion: A review and perspective. Adv. Funct. Mater. 2021, 31, 2011102.
Geng, L. S.; Wang, X. P.; Han, K.; Hu, P.; Zhou, L.; Zhao, Y. L.; Luo, W.; Mai, L. Q. Eutectic electrolytes in advanced metal-ion batteries. ACS Energy Lett. 2022, 7, 247–260.
Zhang, J. N.; Wu, H.; Du, X. F.; Zhang, H.; Huang, L.; Sun, F.; Liu, T. T.; Tian, S. W.; Zhou, L. X.; Hu, S. J. et al. Smart deep eutectic electrolyte enabling thermally induced shutdown toward high-safety lithium metal batteries. Adv. Energy Mater. 2023, 13, 2202529.
Zhang, C. K.; Zhang, L. Y.; Yu, G. H. Eutectic electrolytes as a promising platform for next-generation electrochemical energy storage. Acc. Chem. Res. 2020, 53, 1648–1659.
Wu, W. B.; Liang, Y. H.; Li, D. P.; Bo, Y. Y.; Wu, D.; Ci, L. J.; Li, M. Y.; Zhang, J. H. A competitive solvation of ternary eutectic electrolytes tailoring the electrode/electrolyte interphase for lithium metal batteries. ACS Nano 2022, 16, 14558–14568.
Shao, J. J.; Raidongia, K.; Koltonow, A. R.; Huang, J. X. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability. Nat. Commun. 2015, 6, 7602.
Boisset, A.; Menne, S.; Jacquemin, J.; Balducci, A.; Anouti, M. Deep eutectic solvents based on N-methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 20054–20063.
Boisset, A.; Jacquemin, J.; Anouti, M. Physical properties of a new deep eutectic solvent based on lithium bis[(trifluoromethyl)sulfonyl]imide and N-methylacetamide as superionic suitable electrolyte for lithium ion batteries and electric double layer capacitors. Electrochim. Acta 2013, 102, 120–126.
Liang, Y. H.; Wu, W. B.; Cao, J. W.; Guo, R. T.; Cao, M. M.; Zhang, J. C.; Wang, M.; Yu, W.; Zhang, J. Stable long cycling of small molecular organic acid electrode materials enabled by nonflammable eutectic electrolyte. Small 2022, 18, 2104538.
Liang, Y. H.; Wu, W. B.; Li, D. P.; Wu, H.; Gao, C. C.; Chen, Z. J.; Ci, L. J.; Zhang, J. H. Highly stable lithium metal batteries by regulating the lithium nitrate chemistry with a modified eutectic electrolyte. Adv. Energy Mater. 2022, 12, 2202493.
Song, Y. L.; Yang, L. Y.; Li, J. W.; Zhang, M. Z.; Wang, Y. H.; Li, S. N.; Chen, S. M.; Yang, K.; Xu, K.; Pan, F. Synergistic dissociation-and-trap** effect to promote Li-ion conduction in polymer electrolytes via oxygen vacancies. Small 2021, 17, 2102039.
Yu, L.; Yu, L.; Liu, Q.; Meng, T.; Wang, S.; Hu, X. L. Monolithic task-specific ionogel electrolyte membrane enables high-performance solid-state lithium-metal batteries in wide temperature range. Adv. Funct. Mater. 2022, 32, 2110653.
Wang, S. M.; Chen, Y.; Fang, Q.; Huang, J. J.; Wang, X. F.; Chen, S. M.; Zhang, S. J. Facilitating uniform lithium deposition via nanoconfinement of free amide molecules in solid electrolyte complexion for lithium metal batteries. Energy Storage Mater. 2023, 54, 596–604.
Wang, Z. Y.; Yang, L. S.; Dai, L. X.; Huang, Z. Y.; Wu, K. Y.; Liu, B. L. Scalable production of 2D minerals by polymer intercalation and adhesion for multifunctional applications. Small Methods 2023, 7, 2300529.
Pan, F. S.; Li, Y.; Song, Y. M.; Wang, M. D.; Zhang, Y.; Yang, H.; Wang, H. J.; Jiang, Z. Y. Graphene oxide membranes with fixed interlayer distance via dual crosslinkers for efficient liquid molecular separations. J. Membr. Sci. 2020, 595, 117486.
Li, X. Y.; Li, R. H.; Peng, K.; Fu, L. J.; Zhao, K. P.; Li, H. R.; Peng, J. H.; Wang, L. X. Interlayer functionalization of vermiculite derived silica with hierarchical layered porous structure for stable CO2 adsorption. Chem. Eng. J. 2022, 435, 134875.
Chen, Y.; Yu, D. K.; Fu, L.; Wang, M.; Feng, D. R.; Yang, Y. Z.; Xue, X. M.; Wang, J. F.; Mu, T. C. The dynamic evaporation process of the deep eutectic solvent LiTf2N: N-methylacetamide at ambient temperature. Phys. Chem. Chem. Phys. 2019, 21, 11810–11821.
Wang, C. Z.; Xu, N. K.; Huang, K.; Liu, B.; Zhang, P. H.; Yang, G.; Guo, H. L.; Bai, P.; Mintova, S. Emerging co-synthesis of dimethyl oxalate and dimethyl carbonate using Pd/silicalite-1 catalyst with synergistic interactions of Pd and silanols. Chem. Eng. J. 2023, 466, 143136.
Huang, Z. J.; He, D. D.; Deng, W. H.; **, G. W.; Li, K.; Luo, Y. M. Illustrating new understanding of adsorbed water on silica for inducing tetrahedral cobalt(II) for propane dehydrogenation. Nat. Commun. 2023, 14, 100.
Wu, L. S.; Hu, J. P.; Chen, S. J.; Yang, X. R.; Liu, L.; Foord, J. S.; Pobedinskas, P.; Haenen, K.; Hou, H. J.; Yang, J. K. Lithium nitrate mediated dynamic formation of solid electrolyte interphase revealed by in situ Fourier transform infrared spectroscopy. Electrochim. Acta 2023, 466, 142973.
Liu, Z. E.; Hu, Z. W.; Jiang, X. A.; Wang, X. W.; Li, Z.; Chen, Z. J.; Zhang, Y.; Zhang, S. G. Metal-organic framework confined solvent ionic liquid enables long cycling life quasi-solid-state lithium battery in wide temperature range. Small 2022, 18, 2203011.
Castillo, J.; Santiago, A.; Judez, X.; Garbayo, I.; Coca Clemente, J. A.; Morant-Miñana, M. C.; Villaverde, A.; González-Marcos, J. A.; Zhang, H.; Armand, M. et al. Safe, flexible, and high-performing gelpolymer electrolyte for rechargeable lithium metal batteries. Chem. Mater. 2021, 33, 8812–8821.
Zhu, J. X.; He, S.; Tian, H. Y.; Hu, Y. M.; **n, C.; **e, X. X.; Zhang, L. P.; Gao, J.; Hao, S. M.; Zhou, W. D. et al. The influences of DMF content in composite polymer electrolytes on Li+-conductivity and interfacial stability with Li-metal. Adv. Funct. Mater. 2023, 33, 2301165.
Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X. F.; Wang, T. Y.; Wang, Y. Z.; Kang, F. Y.; Li, B. H.; Wang, G. X. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew. Chem., Int. Ed. 2020, 59, 9134–9142.
Zhou, X. Y.; Li, X. G.; Li, Z.; **e, H. X.; Fu, J. L.; Wei, L.; Yang, H.; Guo, X. Hybrid electrolytes with an ultrahigh Li-ion transference number for lithium-metal batteries with fast and stable charge/discharge capability. J. Mater. Chem. A 2021, 9, 18239–18246.
Du, J. M.; Duan, X. R.; Wang, W. Y.; Li, G. C.; Li, C. H.; Tan, Y. C.; Wan, M. T.; Seh, Z. W.; Wang, L.; Sun, Y. M. Mitigating concentration polarization through acid-base interaction effects for long-cycling lithium metal anodes. Nano Lett. 2023, 23, 3369–3376.
Acknowledgements
The authors would like to acknowledge financial support from National Natural Science Foundation of China (No. U2004199), Joint Foundation for Science and Technology Research & Development Plan of Henan Province (Nos. 222301420003 and 232301420038), China Postdoctoral Science Foundation (No. 2022TQ0293), and Key Science and Technology Project of Henan Province (No. 221100240200-06). Center for advanced analysis and computational science, Zhengzhou University is also highly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, S., Wang, J., Wu, K. et al. Lamellar quasi-solid electrolyte with nanoconfined deep eutectic solvent for high-performance lithium battery. Nano Res. 17, 6176–6183 (2024). https://doi.org/10.1007/s12274-024-6620-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-024-6620-7