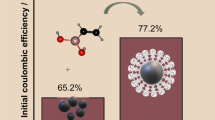
Abstract
The electrochemical performance of hard carbon (HC) materials is closely related to the electrolyte used in the sodium ion batteries (SIBs). Conventional electrolytes carbonate (EC) demonstrates low initial Columbic efficiency (ICE) and poor rate performance, which is one of the main bottlenecks that limits the practical application of HCs. Ether electrolyte (diglyme) was reported to improve the rate performance of HCs. Nevertheless, the underlying mechanism for the excellent rate capability is still lack of in-depth study. In this work, the differences of sodium-ion diffusion between ether and carbonate-base electrolytes in HCs are analyzed layer by layer. Firstly, when sodium-ions are diffused in electrolyte, the diffusion coefficient of sodium-ion in ether electrolyte is about 2.5 times higher than that in ester electrolytes by molecular dynamics (MD) simulation and experimental characterization. Furthermore, when the solvated sodium-ions are diffused into the solid electrolyte interphase (SEI) interface and the HCs material, the enhanced charge transfer kinetics (thin SEI layer (4.6 vs. 12 nm) and low RSEI (1.5 vs. 24 Ω)) at the SEI combined with low desolvation energy (0.248 eV) are responsible for high-rate performance and good cycling stability of HC in ether electrolyte. Therefore, high diffusion coefficient, low desolvation energy, and good interface are the intrinsic reasons for enhanced rate performance in ether electrolyte, which also has guiding significance for the design of other high-rate electrolytes.

Similar content being viewed by others
References
Wang, T. Y.; Su, D. W.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. X. Electrode materials for sodium-ion batteries: Considerations on crystal structures and sodium storage mechanisms. Electrochem. Energy Rev. 2018, 1, 200–237.
Wang, X.; Roy, S.; Shi, Q. H.; Li, Y.; Zhao, Y. F.; Zhang, J. J. Progress in and application prospects of advanced and cost-effective iron (Fe)-based cathode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 1938–1969.
Li, Y.; Shi, Q. H.; Yin, X. P.; Wang, J.; Wang, J.; Zhao, Y. F.; Zhang, J. J. Construction nasicon-type NaTi2(PO4)3 nanoshell on the surface of P2-type Na0.67Co0.2Mn0.8O2 cathode for superior room/low-temperature sodium storage. Chem. Eng. J. 2020, 402, 126181.
Wang, X.; Yin, X. P.; Feng, X. C.; Li, Y.; Dong, X. P.; Shi, Q. H.; Zhao, Y. F.; Zhang, J. J. Rational design of Na0.67Ni0.2Co0.2Mn0.6O2 microsphere cathode material for stable and low temperature sodium ion storage. Chem. Eng. J. 2022, 428, 130990.
Yin, X. P.; Sarkar, S.; Shi, S. S.; Huang, Q. A.; Zhao, H. B.; Yan, L. M.; Zhao, Y. F.; Zhang, J. J. Recent progress in advanced organic electrode materials for sodium-ion batteries: Synthesis, mechanisms, challenges and perspectives. Adv. Funct. Mater. 2020, 30, 1908445.
Shen, L. Y.; Shi, S. S.; Roy, S.; Yin, X. P.; Liu, W. B.; Zhao, Y. F. Recent advances and optimization strategies on the electrolytes for hard carbon and p-based sodium-ion batteries. Adv. Funct. Mater. 2021, 31, 2006066.
Chen, D. Q.; Zhang, W.; Luo, K. Y.; Song, Y.; Zhong, Y. J.; Liu, Y. X.; Wang, G. K.; Zhong, B. H.; Wu, Z. G.; Guo, X. D. Hard carbon for sodium storage: Mechanism and optimization strategies toward commercialization. Energy Environ. Sci. 2021, 14, 2244–2262.
Bai, P. X.; He, Y. W.; Zou, X. X.; Zhao, X. X.; **ong, P. X.; Xu, Y. H. Elucidation of the sodium-storage mechanism in hard carbons. Adv. Energy Mater. 2018, 8, 1703217.
Huang, Y. X.; Zhao, L. Z.; Li, L.; **e, M.; Wu, F.; Chen, R. J. Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: From scientific research to practical application. Adv. Mater. 2019, 31, 1808393.
Wang, P.; Guo, Y. J.; Chen, W. P.; Duan, H.; Ye, H.; Yao, H. R.; Yin, Y. X.; Cao, F. F. Self-supported hard carbon anode from fungus-treated basswood towards sodium-ion batteries. Nano Res. 2023, 16, 3832–3838.
Yin, B.; Liang, S. Q.; Yu, D. D.; Cheng, B. S.; Egun, I. L.; Lin, J. D.; **e, X. F.; Shao, H. Z.; He, H. Y.; Pan, A. Q. Increasing accessible subsurface to improving rate capability and cycling stability of sodium-ion batteries. Adv. Mater. 2021, 33, 2100808.
Chen, X. Y.; Tian, J. Y.; Li, P.; Fang, Y. L.; Fang, Y. J.; Liang, X. M.; Feng, J. W.; Dong, J.; Ai, X. P.; Yang, H. Y.; Cao, Y. An overall understanding of sodium storage behaviors in hard carbons by an “adsorption-intercalation/filling” hybrid mechanism. Adv. Energy Mater. 2022, 12, 2200886.
Au, H.; Alptekin, H.; Jensen, A. C. S.; Olsson, E.; O’Keefe, C. A.; Smith, T.; Crespo-Ribadeneyra, M.; Headen, T. F.; Grey, C. P.; Cai, Q. et al. A revised mechanistic model for sodium insertion in hard carbons. Energy Environ. Sci. 2020, 13, 3469–3479.
Sun, F.; Wang, H.; Qu, Z. B.; Wang, K. F.; Wang, L. J.; Gao, J. H.; Gao, J. M.; Liu, S. Q.; Lu, Y. F. Carboxyl-dominant oxygen rich carbon for improved sodium ion storage: Synergistic enhancement of adsorption and intercalation mechanisms. Adv. Energy Mater. 2021, 11, 2002981.
Song, M. H.; Song, Q.; Zhang, T.; Huo, X. M.; Lin, Z. Z.; Hu, Z. W.; Dong, L.; **, T.; Shen, C.; **e, K. Y. Growing curly graphene layer boosts hard carbon with superior sodium-ion storage. Nano Res., in press, https://doi.org/10.1007/s12274-023-5539-8.
Kim, H.; Hong, J.; Yoon, G.; Kim, H.; Park, K. Y.; Park, M. S.; Yoon, W. S.; Kang, K. Sodium intercalation chemistry in graphite. Energy Environ. Sci. 2015, 8, 2963–2969.
He, Y. W.; Bai, P. X.; Gao, S. Y.; Xu, Y. H. Marriage of an ether-based electrolyte with hard carbon anodes creates superior sodium-ion batteries with high mass loading. ACS Appl. Mater. Interfaces 2018, 10, 41380–41388.
Gong, D. C.; Wei, C. Y.; Liang, Z. W.; Tang, Y. B. Recent advances on sodium-ion batteries and sodium dual-ion batteries: State-of-the-art Na+ host anode materials. Small Sci. 2021, 1, 2100014.
Li, K. K.; Zhang, J.; Lin, D. M.; Wang, D. W.; Li, B. H.; Lv, W.; Sun, S.; He, Y. B.; Kang, F. Y.; Yang, Q. H. et al. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 725.
Zhang, J.; Han, J. W.; Yun, Q. B.; Li, Q.; Long, Y.; Ling, G. W.; Zhang, C.; Yang, Q. H. What is the right carbon for practical anode in alkali metal ion batteries. Small Sci. 2021, 1, 2000063.
Zhen, Y. C.; Sa, R. J.; Zhou, K. Q.; Ding, L. Y.; Chen, Y.; Mathur, S.; Hong, Z. S. Breaking the limitation of sodium-ion storage for nanostructured carbon anode by engineering desolvation barrier with neat electrolytes. Nano Energy 2020, 74, 104895.
Dong, R. Q.; Zheng, L. M.; Bai, Y.; Ni, Q.; Li, Y.; Wu, F.; Ren, H. X.; Wu, C. Elucidating the mechanism of fast Na storage kinetics in ether electrolytes for hard carbon anodes. Adv. Mater. 2021, 33, 2008810.
**a, J. L.; Yan, D.; Guo, L. P.; Dong, X. L.; Li, W. C.; Lu, A. H. Hard carbon nanosheets with uniform ultramicropores and accessible functional groups showing high realistic capacity and superior rate performance for sodium-ion storage. Adv. Mater. 2020, 32, 2000447.
Yin, X. P.; Zhao, Y. F.; Wang, X.; Feng, X. C.; Lu, Z. X.; Li, Y.; Long, H. L.; Wang, J.; Ning, J. Y.; Zhang, J. J. Modulating the graphitic domains of hard carbons derived from mixed pitch and resin to achieve high rate and stable sodium storage. Small 2022, 18, 2105568.
Yin, X. P.; Lu, Z. X.; Wang, J.; Feng, X. C.; Roy, S.; Liu, X. S.; Yang, Y.; Zhao, Y. F.; Zhang, J. J. Enabling fast Na+ transfer kinetics in the whole-voltage-region of hard-carbon anodes for ultrahigh-rate sodium storage. Adv. Mater. 2022, 34, 2109282.
Dai, H. D.; Zeng, Z. Z.; Yang, X. P.; Jiang, M. J. H.; Wang, Y.; Huang, Q. H.; Liu, L. L.; Fu, L. J.; Zhang, P.; Wu, Y. P. Superior potassium storage behavior of hard carbon facilitated by ether-based electrolyte. Carbon 2021, 179, 60–67.
Seh, Z. W.; Sun, J.; Sun, Y. M.; Cui, Y. A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 2015, 1, 449–455.
Ma, M. Y.; Cai, H. R.; Xu, C. L.; Huang, R. Z.; Wang, S. R.; Pan, H. L.; Hu, Y. S. Engineering solid electrolyte interface at nano-scale for high-performance hard carbon in sodium-ion batteries. Adv. Funct. Mater. 2021, 31, 2100278.
Pan, J.; Sun, Y. Y.; Yan, Y. H.; Feng, L.; Zhang, Y. F.; Lin, A. M.; Huang, F. Q.; Yang, J. Revisit electrolyte chemistry of hard carbon in ether for Na storage. JACS Au 2021, 1, 1208–1216.
Plimpton, S. Fast parallel algorithms for short-range molecular dynamic. J. Comp. Phys. 1995, 117, 1–19.
Doherty, B.; Zhong, X.; Gathiaka, S.; Li, B.; Acevedo, O. Revisiting OPLS force field parameters for ionic liquid simulations. J. Chem. Theory Comput. 2017, 13, 6131–6145.
Sambasivarao, S. V.; Acevedo, O. Development of OPLS-AA force field parameters for 68 unique ionic liquids. J. Chem. Theory Comput. 2009, 5, 1038–1050.
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519.
Hoover, W. G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697.
Wang, Z. J.; Wang, Y. Y.; Li, B. H.; Bouwer, J. C.; Davey, K.; Lu, J.; Guo, Z. P. Non-flammable ester electrolyte with boosted stability against Li for high-performance Li metal batteries. Angew. Chem., Int. Ed. 2022, 61, e202206682.
Wang, K. F.; Sun, F.; Wang, H.; Wu, D. Y.; Chao, Y. X.; Gao, J. H.; Zhao, G. B. Altering thermal transformation pathway to create closed pores in coal-derived hard carbon and boosting of Na+ plateau storage for high-performance sodium-ion battery and sodium-ion capacitor. Adv. Funct. Mater. 2022, 32, 2203725.
Zhang, J.; Wang, D. W.; Lv, W.; Zhang, S. W.; Liang, Q. H.; Zheng, D. Q.; Kang, F. Y.; Yang, Q. H. Achieving superb sodium storage performance on carbon anodes through an ether-derived solid electrolyte interphase. Energy Environ. Sci. 2017, 10, 370–376.
Kang, M. L.; Wu, Y. Y.; Huang, X.; Zhou, K. Q.; Huang, Z. G.; Hong, Z. S. Engineering of a TiO2 anode toward a record high initial Coulombic efficiency enabling high-performance low-temperature Na-ion hybrid capacitors. J. Mater. Chem. A 2018, 6, 22840–22850.
Pan, K. H.; Lu, H. Y.; Zhong, F. P.; Ai, X. P.; Yang, H. X.; Cao, Y. L. Understanding the electrochemical compatibility and reaction mechanism on Na metal and hard carbon anodes of PC-based electrolytes for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 39651–39660.
Huang, J. Q.; Guo, X. Y.; Du, X. Q.; Lin, X. Y.; Huang, J. Q.; Tan, H.; Zhu, Y.; Zhang, B. Nanostructures of solid electrolyte interphases and their consequences for microsized Sn anodes in sodium ion batteries. Energy Environ. Sci. 2019, 12, 1550–1557.
Zhang, W. G.; Zeng, F. H.; Huang, H. J.; Yu, Y.; Xu, M. Q.; **ng, L. D.; Li, W. S. Enhanced interphasial stability of hard carbon for sodium-ion battery via film-forming electrolyte additive. Nano Res. 2023, 16, 3823–3831.
Wu, Z. R.; Zou, J.; Shabanian, S.; Golovin, K.; Liu, J. The roles of electrolyte chemistry in hard carbon anode for potassium-ion batteries. Chem. Eng. J. 2022, 427, 130972.
Lee, M. E.; Lee, S. M.; Choi, J.; Jang, D.; Lee, S.; **, H. J.; Yun, Y. S. Electrolyte-dependent sodium ion transport behaviors in hard carbon anode. Small 2020, 16, 2001053.
Gachot, G.; Grugeon, S.; Armand, M.; Pilard, S.; Guenot, P.; Tarascon, J. M.; Laruelle, S. Deciphering the multi-step degradation mechanisms of carbonate-based electrolyte in Li batteries. J. Power Sources 2008, 178, 409–421.
Eshetu, G. G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R. J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the electrolyte salt anion on the solid electrolyte interphase formation in sodium ion batteries. Nano Energy 2019, 55, 327–340.
Le, P. M. L.; Vo, T. D.; Pan, H. L.; **, Y.; He, Y.; Cao, X.; Nguyen, H. V.; Engelhard, M. H.; Wang, C. M.; **ao, J. et al. Excellent cycling stability of sodium anode enabled by a stable solid electrolyte interphase formed in ether-based electrolytes. Adv. Funct. Mater. 2020, 30, 2001151.
Li, K.; Galle Kankanamge, S. R.; Weldeghiorghis, T. K.; Jorn, R.; Kuroda, D. G.; Kumar, R. Predicting ion association in sodium electrolytes: A transferable model for investigating glymes. J. Phy. Chem. C 2018, 122, 4747–4756.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 22179077, 51774251, and 21908142), Shanghai Science and Technology Commission’s “2020 Science and Technology In-novation Action Plan” (No. 20511104003), and Natural Science Foundation in Shanghai (No. 21ZR1424200). The authors also thank Shiyanjia Lab (https://www.shiyanjia.com) for the XPS measurement.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yin, X., Wang, Z., Liu, Y. et al. Insight into the influence of ether and ester electrolytes on the sodium-ion transportation kinetics for hard carbon. Nano Res. 16, 10922–10930 (2023). https://doi.org/10.1007/s12274-023-5793-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5793-9