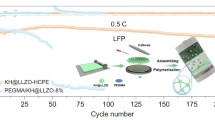
Abstract
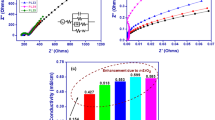
Solid-state lithium batteries using composite polymer electrolytes (CPEs) have attracted much attention owing to their higher safety compared to liquid electrolytes and flexibility compared to ceramic electrolytes. However, their unsatisfactory lithium-ion conductivity still limits their development. Herein, a high ion conductive CPE with multiple continuous lithium pathways is designed. This new electrolyte consists of poly(vinylidene fluorideco-hexafluoropropylene) (PVDF-HFP) and lithiated X type zeolite (Li-X), which possesses a high ionic conductivity (1.98 × 10−4 S/cm), high lithium transference number \((t_{\text{Li}^{+}}=0.55)\), wide electrochemical window (4.7 V), and excellent stability against the lithium anode. Density functional theory (DFT) calculation confirms that the Lewis acid sites in zeolite can graft with N,N-dimethylformamide (DMF) and PVDF-HFP chains, resulting in decreased crystallinity of polymer and providing rapid Li+ transmission channels. When used in a full cell, the solid Li∣Li-X-3%∣LiFePO4 cell displays excellent cycling stability and rate performance at room temperature and 60 °C. Furthermore, pouch cells with the Li-X-3% electrolyte exhibit brilliant safety under extreme conditions, such as folding and cutting. Thus, this proposed zeolite-PVDF-HFP CPE represents a promising potential in the application of making a safer, higher performing, and flexible solid-state lithium battery.

Similar content being viewed by others
References
Chen, X.; Huang, H. J.; Pan, L.; Liu, T.; Niederberger, M. Fully integrated design of a stretchable solid-state lithium-ion full battery. Adv. Mater. 2019, 31, 1904648.
Mathies, L.; Diddens, D.; Dong, D. P.; Bedrov, D.; Leipner, H. Transport mechanism of lithium ions in non-coordinating P(VdF-HFP) copolymer matrix. Solid State Ionics 2020, 357, 115497.
Cheng, J.; Hou, G. M.; Chen, Q.; Li, D. P.; Li, K. K.; Yuan, Q. H.; Wang, J. J.; Ci, L. J. Sheet-like garnet structure design for upgrading PEO-based electrolyte. Chem. Eng. J. 2022, 429, 132343.
Ke, R. H.; Du, L. L.; Han, B.; Xu, H. L.; Meng, H.; Zeng, H. B.; Zheng, Z. J.; Deng, Y. H. Biobased self-growing approach toward tailored, integrated high-performance flexible lithium-ion battery. Nano Lett. 2022, 22, 9327–9334.
Lian, P. J.; Zhao, B. S.; Zhang, L. Q.; Xu, N.; Wu, M. T.; Gao, X. P. Inorganic sulfide solid electrolytes for all-solid-state lithium secondary batteries. J. Mater. Chem. A 2019, 7, 20540–20557.
Guan, D. H.; Wang, X. X.; Li, F.; Zheng, L. J.; Li, M. L.; Wang, H. F.; Xu, J. J. All-solid-state photo-assisted Li-CO2 battery working at an ultra-wide operation temperature. ACS Nano 2022, 16, 12364–12376.
MacGlashan, G. S.; Andreev, Y. G.; Bruce, P. G. Structure of the polymer electrolyte poly(ethylene oxide)6: LiAsF6. Nature 1999, 398, 792–794.
Chan, C. H.; Kammer, H. W.; Sim, L. H.; Yusoff, S. N. H. M.; Hashifudin, A.; Winie, T. Conductivity and dielectric relaxation of Li salt in poly(ethylene oxide) and epoxidized natural rubber polymer electrolytes. Ionics 2014, 20, 189–199.
Li, C.; Guo, Z. Y.; Yang, B. C.; Liu, Y.; Wang, Y. G.; **a, Y. Y. A rechargeable Li-CO2 battery with a gel polymer electrolyte. Angew. Chem., Int. Ed. 2017, 56, 9126–9130.
Cheng, Z. W.; Liu, T.; Zhao, B.; Shen, F.; **, H. Y.; Han, X. G. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater. 2021, 34, 388–416.
Li, S.; Zhang, S. Q.; Shen, L.; Liu, Q.; Ma, J. B.; Lv, W.; He, Y. B.; Yang, Q. H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088.
Kong, X.; Rudnicki, P. E.; Choudhury, S.; Bao, Z. N.; Qin, J. Dendrite suppression by a polymer coating: A coarse-grained molecular study. Adv. Funct. Mater. 2020, 30, 1910138.
Elashmawi, I. S.; Gaabour, L. H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110.
Du, C. H.; Zhu, B. K.; Xu, Y. Y. The effects of quenching on the phase structure of vinylidene fluoride segments in PVDF-HFP copolymer and PVDF-HFP/PMMA blends. J. Mater. Sci. 2006, 41, 417–421.
Ye, F.; Liao, K. M.; Ran, R.; Shao, Z. P. Recent advances in filler engineering of polymer electrolytes for solid-state Li-ion batteries: A review. Energy Fuels 2020, 34, 9189–9207.
Zhang, X.; Liu, T.; Zhang, S. F.; Huang, X.; Xu, B. Q.; Lin, Y. H.; Xu, B.; Li, L. L.; Nan, C. W.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785.
Wang, Z. X.; Huang, X. J.; Chen, L. Q. Understanding of effects of nano-Al2O3 particles on ionic conductivity of composite polymer electrolytes. Electrochem. Solid-State Lett. 2003, 6, E40.
Croce, F.; Appetecchi, G. B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458.
Jayathilaka, P. A. R. D.; Dissanayake, M. A. K. L.; Albinsson, I.; Mellander, B. E. Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochim. Acta 2002, 47, 3257–3268.
Chan, C. K.; Yang, T.; Weller, J. M. Nanostructured garnet-type Li7La3Zr2O12: Synthesis, properties, and opportunities as electrolytes for Li-ion batteries. Electrochim. Acta 2017, 253, 268–280.
Xu, D.; Su, J. M.; **, J.; Sun, C.; Ruan, Y. D.; Chen, C. H.; Wen, Z. Y. In situ generated fireproof gel polymer electrolyte with Li6.4Ga0.2La3Zr2O12 as initiator and ion-conductive filler. Adv. Energy Mater. 2019, 9, 1900611.
Huang, Z. Y.; Chen, L. H.; Huang, B.; Xu, B. Y.; Shao, G.; Wang, H. L.; Li, Y. T.; Wang, C. A. Enhanced performance of Li6.4La3Zr1.4Ta0.6O12 solid electrolyte by the regulation of grain and grain boundary phases. ACS Appl. Mater. Interfaces 2020, 12, 56118–56125.
Mo, Y. F.; Ong, S. P.; Ceder, G. First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem. Mater. 2012, 24, 15–17.
Suzuki, K.; Kato, D.; Hara, K.; Yano, T. A.; Hirayama, M.; Hara, M.; Kanno, R. Composite sulfur electrode prepared by high-temperature mechanical milling for use in an all-solid-state lithium-sulfur battery with a Li3.25Ge0.25P0.75S4 electrolyte. Electrochim. Acta 2017, 258, 110–115.
Zhang, Z.; Huang, Y.; Gao, H.; Li, C.; Hang, J. X.; Liu, P. B. MOF-derived multifunctional filler reinforced polymer electrolyte for solidstate lithium batteries. J. Energy Chem. 2021, 60, 259–271.
Zhang, G.; Hong, Y. L.; Nishiyama, Y.; Bai, S. Y.; Kitagawa, S.; Horike, S. Accumulation of glassy poly(ethylene oxide) anchored in a covalent organic framework as a solid-state Li+ electrolyte. J. Am. Chem. Soc. 2019, 141, 1227–1234.
Barbosa, J. C.; Gonçalves, R.; Costa, C. M.; de Zea Bermudez, V.; Fidalgo-Marijuan, A.; Zhang, Q.; Lanceros-Méndez, S. Metal-organic frameworks and zeolite materials as active fillers for lithium-ion battery solid polymer electrolytes. Mater. Adv. 2021, 2, 3790–3805.
Wang, X. X.; Chi, X. W.; Li, M. L.; Guan, D. H.; Miao, C. L.; Xu, J. J. Metal-organic frameworks derived electrolytes build multiple wetting interfaces for integrated solid-state lithium-oxygen battery. Adv. Funct. Mater. 2022, 32, 2113235.
Wang, X. X.; Chi, X. W.; Li, M. L.; Guan, D. H.; Miao, C. L.; Xu, J. J. An integrated solid-state lithium-oxygen battery with highly stable anionic covalent organic frameworks electrolyte. Chem 2023, 9, 394–410.
Li, Z. G.; Wu, X. H.; Yu, X. Y.; Zhou, S. Y.; Qiao, Y.; Zhou, H. S.; Sun, S. G. Long-life aqueous Zn-I2 battery enabled by a low-cost multifunctional zeolite membrane separator. Nano Lett. 2022, 22, 2538–2546.
Chi, X. W.; Li, M. L.; Di, J. C.; Bai, P.; Song, L. N.; Wang, X. X.; Li, F.; Liang, S.; Xu, J. J.; Yu, J. H. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021, 592, 551–557.
Cui, Y. H.; Zhao, Q. H.; Wu, X. J.; Chen, X.; Yang, J. L.; Wang, Y. T.; Qin, R. Z.; Ding, S. X.; Song, Y. L.; Wu, J. W. et al. An interface-bridged organic—inorganic layer that suppresses dendrite formation and side reactions for ultra-long-life aqueous zinc metal anodes. Angew. Chem., Int. Ed. 2020, 59, 16594–16601.
Zheng, L. J.; Bai, P.; Yan, W. F.; Li, F.; Wang, X. X.; Xu, J. J. In situ construction of glass-fiber-directed zeolite microtube woven separator for ultra-high-capacity lithium-oxygen batteries. Matter 2023, 6, 142–157.
Huang, Y. F.; Gu, T.; Rui, G. C.; Shi, P. R.; Fu, W. B.; Chen, L.; Liu, X. T.; Zeng, J. P.; Kang, B. H.; Yan, Z. C. et al. A relaxor ferroelectric polymer with an ultrahigh dielectric constant largely promotes the dissociation of lithium salts to achieve high ionic conductivity. Energy Environ. Sci. 2021, 14, 6021–6029.
Mi, J. S.; Ma, J. B.; Chen, L. K.; Lai, C.; Yang, K.; Biao, J.; **a, H. Y.; Song, X.; Lv, W.; Zhong, G. M. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling highvoltage lithium metal solid-state batteries. Energy Storage Mater. 2022, 48, 375–383.
Liu, Q. Y.; Yang, G. J.; Li, X. Y.; Zhang, S. M.; Chen, R. J.; Wang, X. F.; Gao, Y. R.; Wang, Z. X.; Chen, L. Q. Polymer electrolytes based on interactions between [solvent-Li+] complex and solvent-modified polymer. Energy Storage Mater. 2022, 51, 443–452.
Zhang, X.; Han, J.; Niu, X. F.; **n, C. Z.; Xue, C. J.; Wang, S.; Shen, Y.; Zhang, L.; Li, L. L.; Nan, C. W. High cycling stability for solid-state Li metal batteries via regulating solvation effect in poly(vinylidene fluoride)-based electrolytes. Batter. Supercaps 2020, 3, 876–883.
Lang, J. L.; Long, Y. Z.; Qu, J. L.; Luo, X. Y.; Wei, H. H.; Huang, K.; Zhang, H. T.; Qi, L. H.; Zhang, Q. F.; Li, Z. C. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2019, 16, 85–90.
Ding, Z. Y.; Tang, Q. M.; Liu, Y. C.; Yao, P. H.; Liu, C.; Liu, X. J.; Wu, J. W.; Lavorgna, M. Integrate multifunctional ionic sieve lithiated X zeolite-ionic liquid electrolyte for solid-state lithium metal batteries with ultralong lifespan. Chem. Eng. J. 2022, 433, 133522.
**, Z. N.; Lei, D.; Wang, Y.; Wu, L. K.; Hu, N. Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films. Nanotechnol. Rev. 2021, 10, 1009–1017.
Tian, X. Z.; Jiang, X. Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Wu, L. K.; Yuan, W. F.; Hu, N.; Wang, Z. C.; Chen, C. L.; Qiu, J. H.; Ying, J.; Li, Y. Improved piezoelectricity of PVDF-HFP/carbon black composite films. J. Phys. D: Appl. Phys. 2014, 47, 135302.
Lee, J. Y.; Chung, P. H.; Yeh, S. C.; Yu, T. Y.; Lee, W. Y.; Wu, N. L.; Jeng, R. J. Tough polymer electrolyte with an intrinsically stabilized interface with Li metal for all-solid-state lithium-ion batteries. J. Phys. Chem. C 2021, 125, 26339–26347.
Mishra, R.; Singh, S. K.; Gupta, H.; Tiwari, R. K.; Meghnani, D.; Patel, A.; Tiwari, A.; Tiwari, V. K.; Singh, R. K. Polar β-phase PVdF-HFP-based freestanding and flexible gel polymer electrolyte for better cycling stability in a Na battery. Energy Fuels 2021, 35, 15153–15165.
Tohluebaji, N.; Putson, C.; Muensit, N. High electromechanical deformation based on structural beta-phase content and electrostrictive properties of electrospun poly(vinylidene fluoride-hexafluoropropylene) nanofibers. Polymers (Basel) 2019, 11, 1817.
Yao, P. C.; Zhu, B.; Zhai, H. W.; Liao, X. B.; Zhu, Y. X.; Xu, W. H.; Cheng, Q.; Jayyosi, C.; Li, Z.; Zhu, J. et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Lett. 2018, 18, 6113–6120.
Yang, K.; Chen, L. K.; Ma, J. B.; Lai, C.; Huang, Y. F.; Mi, J. S.; Biao, J.; Zhang, D. F.; Shi, P. R.; **a, H. X. et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries. Angew. Chem., Int. Ed. 2021, 60, 24668–24675.
Liu, L.; Zhang, D. C.; Zhao, J. W.; Shen, J. D.; Li, F. K.; Yang, Y.; Liu, Z. B.; He, W. X.; Zhao, W. M.; Liu, J. Synergistic effect of lithium salts with fillers and solvents in composite electrolytes for superior room-temperature solid-state lithium batteries. ACS Appl. Energy Mater. 2022, 5, 2484–2494.
Gonçalves, R.; Miranda, D.; Almeida, A. M.; Silva, M. M.; Meseguer-Dueñas, J. M.; Ribelles, J. L. G.; Lanceros-Méndez, S.; Costa, C. M. Solid polymer electrolytes based on lithium bis(trifluoromethanesulfonyl)imide/poly(vinylidene fluoride-co-hexafluoropropylene) for safer rechargeable lithium-ion batteries. Sustain. Mater. Technol. 2019, 21, e00104.
Fujii, K.; Wakamatsu, H.; Todorov, Y.; Yoshimoto, N.; Morita, M. Structural and electrochemical properties of Li ion solvation complexes in the salt-concentrated electrolytes using an aprotic donor solvent, N,N-dimethylformamide. J. Phys. Chem. C 2016, 120, 17196–17204.
Xu, F. L.; Deng, S. G.; Guo, Q. Y.; Zhou, D.; Yao, X. Y. Quasi-ionic liquid enabling single-phase poly(vinylidene fluoride)-based polymer electrolytes for solid-state LiNi0.6Co0.2Mn0.2O2∣ ∣Li batteries with rigid-flexible coupling interphase. Small Methods 2021, 5, 2100262.
Jacob, M.; Arof, A. K. FTIR studies of DMF plasticized polyvinyledene fluoride based polymer electrolytes. Electrochim. Acta 2000, 45, 1701–1706.
Rey, I.; Johansson, P.; Lindgren, J.; Lassègues, J. C.; Grondin, J.; Servant, L. Spectroscopic and theoretical study of (CF3SO2)2N−(TFSI−) and (CF3SO2)2NH(HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258.
Li, Z. X.; Zhang, S. Z.; Jiang, Z.; Cai, D.; Gu, C. D.; Tu, J. P. Deep eutectic solvent-immobilized PVDF-HFP eutectogel as solid electrolyte for safe lithium metal battery. Mater. Chem. Phys. 2021, 267, 124701.
Aravindan, V.; Vickraman, P. Characterization of SiO2 and Al2O3 incorporated PVdF-HFP based composite polymer electrolytes with LiPF3(CF3CF2)3. J. Appl. Polym. Sci. 2008, 108, 1314–1322.
Zuo, X. X.; Ma, X. D.; Wu, J. H.; Deng, X.; **ao, X.; Liu, J. S.; Nan, J. M. Self-supporting ethyl cellulose/poly(vinylidene fluoride) blended gel polymer electrolyte for 5 V high-voltage lithium-ion batteries. Electrochim. Acta 2018, 271, 582–590.
Wang, W.; Yuan, Y.; Wang, J. L.; Zhang, Y.; Liao, C.; Mu, X. W.; Sheng, H. B.; Kan, Y. C.; Song, L.; Hu, Y. Enhanced electrochemical and safety performance of lithium metal batteries enabled by the atom layer deposition on PVDF-HFP separator. ACS Appl. Energy Mater. 2019, 2, 4167–4174.
Poole, J. L.; Riding, K. A.; Folliard, K. J.; Juenger, M. C. G.; Schindler, A. K. Methods for calculating activation energy for Portland cement. ACI Mater. J. 2007, 104, 303–311.
Sun, Z. J.; Liu, L.; Yang, B.; Li, Q. R.; Wu, B.; Zhao, J. T.; Ma, L.; Liu, Y.; An, H. L. Preparation and ion conduction of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte films using radio frequency sputtering. Solid State Ionics 2020, 346, 115224.
Zhang, X.; Wang, S.; Xue, C. J.; **n, C. Z.; Lin, Y. H.; Shen, Y.; Li, L. L.; Nan, C. W. Self-suppression of lithium dendrite in all-solidstate lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes. Adv. Mater. 2019, 31, 1806082.
Chen, L. K.; Gu, T.; Ma, J. B.; Yang, K.; Shi, P. R.; Biao, J.; Mi, J. S.; Liu, M.; Lv, W.; He, Y. B. In situ construction of Li3N-enriched interface enabling ultra-stable solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries. Nano Energy 2022, 100, 107470.
Acknowledgements
This work was supported by the Stable Supporting Fund of Shenzhen (No. GXWD20201230155427003-20200728114835006).
The authors would like to thank the shiyanjia lab (https://www.shiyanjia.com) for the DFT calculation support.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Ding, Z., Tang, Q., Zhang, Q. et al. A flexible solid polymer electrolyte enabled with lithiated zeolite for high performance lithium battery. Nano Res. 16, 9443–9452 (2023). https://doi.org/10.1007/s12274-023-5658-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5658-2