Abstract
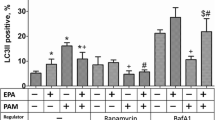
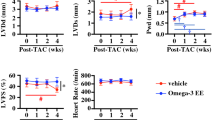
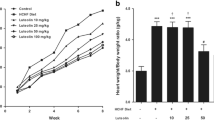
Eicosapentaenoic acid (EPA) reduces the risk of ischemic heart diseases and is a component of mitochondria. We herein investigated whether dietary EPA mediated mitochondrial fatty acid compositions, dynamics, and functions, resulting in the attenuation of cardiac remodeling after myocardial infarction (MI). The coronary artery of male rats was ligated to induce MI, and they were then treated with or without EPA (1000 mg/kg/day) for 12 weeks. The EPA treatment improved left ventricular systolic function and increased the mitochondrial content of EPA in the non-infarct region 12 weeks after MI. The content of ATP and mitochondrial complex II, III, and IV activities decreased after MI but were maintained by the EPA treatment in association with the preservation of optic atrophy 1, a mitochondrial fusion protein. The present results suggest that dietary EPA increased the mitochondrial content of EPA and preserved the expression of mitochondrial fusion proteins and energy metabolism, which attenuated left ventricular remodeling after MI.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data used to support the present results are available upon request from the corresponding author.
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-Linolenic acid
- ATP:
-
Adenosine triphosphate
- BSA:
-
Bovine serum albumin
- BW:
-
Body weight
- CHF:
-
Chronic heart failure
- DGLA:
-
Dihomo-γ-linolenic acid
- DHA:
-
Docosapentaenoic acid
- Drp-1:
-
Dynamin-related protein 1
- EGTA:
-
Ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- EPA:
-
Eicosapentaenoic acid
- GC-MS:
-
Gas chromatography–mass spectrometry
- GLA:
-
γ-Linolenic acid
- HE:
-
Hematoxylin-eosin
- HW:
-
Heart weight
- LV:
-
Left ventricular
- LVEDd:
-
Left ventricular end-diastolic diameter
- LVEDs:
-
Left ventricular end-systolic diameter
- LVFS:
-
Left ventricular fractional shortening
- LVW:
-
Left ventricular weight
- LW:
-
Lung weight
- MI:
-
Myocardial infarction
- MOPS:
-
Morpholinepropanesulfonic acid
- OPA1:
-
Optic atrophy 1
- PUFA:
-
Polyunsaturated fatty acids
- RBC:
-
Red blood cell
- TA:
-
Tridecanoic acid
- TG:
-
Triglyceride
- TTC:
-
Triphenyl tetrazolium chloride
References
Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8.
Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30.
GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55.
Heydari B, Abdullah S, Pottala JV, et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL randomized clinical trial. Circulation. 2016;134:378–91.
Nodari S, Triggiani M, Campia U, et al. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:870–9.
Ajith TA, Jayakumar TG. Omega-3 fatty acids in coronary heart disease: recent updates and future perspectives. Clin Exp Pharmacol Physiol. 2019;46:11–8.
Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–9.
Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26.
Trial Investigators ORIGIN, Bosch J, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–18.
Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–8.
Takamura M, Kurokawa K, Ootsuji H, et al. Long-term administration of eicosapentaenoic acid improves post-myocardial infarction cardiac remodeling in mice by regulating macrophage polarization. J Am Heart Assoc. 2017;6(2):e004560.
Duda MK, O’Shea KM, Lei B, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–10.
Duda MK, O’Shea KM, Tintinu A, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–27.
Chen J, Shearer GC, Chen Q, et al. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123:584–93.
Li Q, Yu Q, Na R, Liu B. Omega-3 polyunsaturated fatty acids prevent murine dilated cardiomyopathy by reducing oxidative stress and cardiomyocyte apoptosis. Exp Ther Med. 2017;14:6152–8.
Gao JY, Yasuda S, Tsuburaya R, et al. Long-term treatment with eicosapentaenoic acid ameliorates myocardial ischemia-reperfusion injury in pigs in vivo. -Involvement of Rho-kinase pathway inhibition-. Circ J. 2011;75:1843–51.
Anderson EJ, Thayne K, Harris M, et al. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem J. 2012;441:359–66.
Ogita H, Node K, Asanuma H, et al. Eicosapentaenoic acid reduces myocardial injury induced by ischemia and reperfusion in rabbit hearts. J Cardiovasc Pharmacol. 2003;41:964–9.
Monteiro JP, Oliveira PJ, Jurado AS. Mitochondrial membrane lipid remodeling in pathophysiology: a new target for diet and therapeutic interventions. Prog Lipid Res. 2013;52:513–28.
Sharov VG, Todor AV, Silverman N, et al. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–7.
Rosca MG, Vazquez EJ, Kerner J, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–9.
Youle RJ. van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5.
Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–70.
Griparic L, van der Bliek AM. The many shapes of mitochondrial membranes. Traffic. 2001;2:235–44.
Ong SB, Subrayan S, Lim SY, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22.
Li XX, Tsoi B, Li YF, et al. Cardiolipin and its different properties in mitophagy and apoptosis. J Histochem Cytochem. 2015;63:301–11.
Ito S, Sano Y, Nagasawa K, et al. Highly purified eicosapentaenoic acid ameliorates cardiac injury and adipose tissue inflammation in a rat model of metabolic syndrome. Obes Sci Pract. 2016;2:318–29.
Kobara M, Tatsumi T, Matoba S, et al. Effect of ischemic preconditioning on mitochondrial oxidative phosphorylation and high energy phosphates in rat hearts. J Mol Cell Cardiol. 1996;28:417–28.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31.
Higashihara E, Nutahara E, Horie S, et al. The effect of eicosapentaenoic acid on renal function and volume in patients with ADPKD. Nephrol Dial Transplant. 2008;23:2847–52.
Zeghichi-Hamri S, de Lorgeril M, Salen P, et al. Protective effect of dietary n-3 polyunsaturated fatty acids on myocardial resistance to ischemia-reperfusion injury in rats. Nutr Res. 2010;30:849–57.
Birk AV, Chao WM, Bracken C, et al. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171:2017–28.
McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–85.
Johnson ML, Lalia AZ, Dasari S, et al. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell. 2015;14:734–43.
Sun R, Wang X, Liu Y, **a M. Dietary supplementation with fish oil alters the expression levels of proteins governing mitochondrial dynamics and prevents high-fat diet-induced endothelial dysfunction. Br J Nutr. 2014;112:145–53.
Dorn GW 2nd. Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013;1833:233–41.
Jiang HK, Wang YH, Sun L, et al. Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction: roles of mitochondrial network dynamics. Int J Mol Sci. 2014;15:5304–22.
Wang F, Yang J, Sun J, et al. Testosterone replacement attenuates mitochondrial damage in a rat model of myocardial infarction. J Endocrinol. 2015;225:101–11.
Piquereau J, Caffin F, Novotova M, et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94:408–17.
Varanita T, Soriano ME, Romanello V, et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–44.
Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101:15927–32.
Shysh AM, Nagibin VS, Kaplinskii SP, Dosenko VE. N-3 long chain polyunsaturated fatty acids increase the expression of PPARγ-target genes and resistance of isolated heart and cultured cardiomyocytes to ischemic injury. Pharmacol Rep. 2016;68:1133–9.
Anusree SS, Nisha VM, Priyanka A. Raghu KG (2015) Insulin resistance by TNF-α is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol Cell Endocrinol. 2015;413:120–8.
Acknowledgements
We would like to thank Mochida Pharmaceutical Co., Ltd. (Tokyo, Japan) for supplying EPA.
Funding
This work was supported, in part, by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 24591102.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Miyuki Kobara designed and performed the experiments and wrote the first draft of the manuscript. Tatsuya Shiraishi performed the experiments and data analysis. Kazuki Noda performed the GC–MS measurement and analysis. Hiroe Toba performed the data analysis. Tetsuo Nakata contributed to the conception of the study and drafting of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The protocol was approved by the Bioethics Committee of Kyoto Pharmaceutical University.
Consent to Participate
No human studies were performed by the authors for this article.
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Guo** Li oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobara, M., Shiraishi, T., Noda, K. et al. Eicosapentaenoic Acid Preserves Mitochondrial Quality and Attenuates Cardiac Remodeling After Myocardial Infarction in Rats. J. of Cardiovasc. Trans. Res. 16, 816–827 (2023). https://doi.org/10.1007/s12265-023-10363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10363-z