Abstract
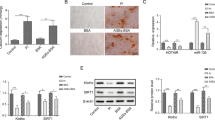
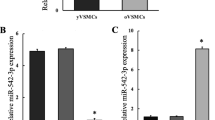
Atherosclerosis is the most common arterial disease and is closely related to vascular calcification. CircHIPK3 has been implicated in atherosclerosis development, but the possible downstream regulatory mechanisms remain unclear. The levels of circHIPK3, miR-106a and MFN2 in tissues and blood samples of patients with atherosclerosis were detected by RT-qPCR. The levels of circHIPK3, miR-106a and MFN2 were detected by RT-qPCR and the expression levels of MFN2, osteogenic and cartilage differentiation marker proteins were detected by western blot in vitro. ALP staining, Alizarin Red staining, and calcium content detection evaluated the degree of osteogenic differentiation of cells. Alcian blue staining detected the level of cell cartilage differentiation. Luciferase detected the targeting relationship between circHIPK3 and miR-106a-5p, as well as miR-106a-5p and MFN2. CircHIPK3 and MFN2 were low expressed and miR-106a-5p was highly expressed in tissues and blood samples of patients with atherosclerosis, as well as vascular smooth muscle cell (VSMC) with osteogenic and cartilage differentiation. Overexpression of circHIPK3 reduced the cell mineralization and calcium content. Overexpression of circHIPK3 inhibited osteogenic differentiation by decreasing ALP activity, RUNX2, and OPG expression, and increasing SM22α and SMA level. What’s more, overexpression of circHIPK3 decreased the chondrogenic differentiation by inhibiting the protein level of SOX9, aggrecan, and collagen II. CircHIPK3 targeted miR-106a-5p and miR-106a-5p targeted MFN2. MiR-106a-5p overexpression or MFN2 depletion repressed the effect of circHIPK3 overexpression on VSMC calcification. CircHIPK3 regulated osteogenic and cartilage differentiation of VSMC via miR-106a-5p/MFN2 axis, indicating a target for treating vascular calcification.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALP:
-
Alkaline phosphatase
- CircRNA:
-
Circular RNA
- FBS:
-
Fetal bovine serum
- MiRNA:
-
MicroRNA
- MFN2:
-
Mitochondrial fusion protein 2
- PBS:
-
Phosphate-buffered solution
- RIP:
-
RNA binding protein immunoprecipitation
- RT-qPCR:
-
Real-time fluorescence quantitative PCR
- VSMC:
-
Vascular smooth muscle cells
References
Banerjee, S., Bagheri, M., Sandfort, V., Ahlman, M. A., Malayeri, A. A., Bluemke, D. A., et al. (2019). Vascular calcification in patients with large-vessel vasculitis compared to patients with hyperlipidemia. Seminars in Arthritis and Rheumatism, 48(6), 1068–1073. https://doi.org/10.1016/j.semarthrit.2018.09.001
Zhu, Y., **an, X., Wang, Z., Bi, Y., Chen, Q., Han, X., et al. (2018). Research progress on the relationship between atherosclerosis and inflammation. Biomolecules, 8(3). https://doi.org/10.3390/biom8030080.
Wu, M., Rementer, C., & Giachelli, C. M. (2013). Vascular calcification: An update on mechanisms and challenges in treatment. Calcified Tissue International, 93(4), 365–373. https://doi.org/10.1007/s00223-013-9712-z
Chin, D. D., Wang, J., Mel de Fontenay, M., Plotkin, A., Magee, G. A., & Chung, E. J. (2019). Hydroxyapatite-binding micelles for the detection of vascular calcification in atherosclerosis. Journal of Materials Chemistry B, 7(41), 6449–57. https://doi.org/10.1039/c9tb01918a
Zhu, L., Zhang, N., Yan, R., Yang, W., Cong, G., Yan, N., et al. (2019). Hyperhomocysteinemia induces vascular calcification by activating the transcription factor RUNX2 via Kruppel-like factor 4 up-regulation in mice. Journal of Biological Chemistry, 294(51), 19465–19474. https://doi.org/10.1074/jbc.RA119.009758
Zhang, F., Zhang, R., Zhang, X., Wu, Y., Li, X., Zhang, S., et al. (2018). Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging (Albany NY), 10(9), 2266–2283. https://doi.org/10.18632/aging.101541
Zhang, H. D., Jiang, L. H., Sun, D. W., Hou, J. C., & Ji, Z. L. (2018). CircRNA: A novel type of biomarker for cancer. Breast Cancer, 25(1), 1–7. https://doi.org/10.1007/s12282-017-0793-9
Wang, S. W., Liu, Z., & Shi, Z. S. (2018). Non-coding RNA in acute ischemic stroke: Mechanisms, biomarkers and therapeutic targets. Cell Transplantation, 27(12), 1763–1777. https://doi.org/10.1177/0963689718806818
Yang, F., Chen, Y., Xue, Z., Lv, Y., Shen, L., Li, K., et al. (2020). High-Throughput sequencing and exploration of the lncRNA-circRNA-miRNA-mRNA network in type 2 diabetes mellitus. BioMed Research International, 2020, 8162524. https://doi.org/10.1155/2020/8162524
Chen, X., Mao, R., Su, W., Yang, X., Geng, Q., Guo, C., et al. (2020). Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKalpha signaling in STK11 mutant lung cancer. Autophagy, 16(4), 659–671. https://doi.org/10.1080/15548627.2019.1634945
Zhang, Y., Li, C., Liu, X., Wang, Y., Zhao, R., Yang, Y., et al. (2019). circHIPK3 promotes oxaliplatin-resistance in colorectal cancer through autophagy by sponging miR-637. eBioMedicine, 48, 277–288. https://doi.org/10.1016/j.ebiom.2019.09.051
Zheng, Q., Bao, C., Guo, W., Li, S., Chen, J., Chen, B., et al. (2016). Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nature Communications, 7, 11215. https://doi.org/10.1038/ncomms11215
Si, X., Zheng, H., Wei, G., Li, M., Li, W., Wang, H., et al. (2020). circRNA Hipk3 induces cardiac regeneration after myocardial infarction in mice by binding to Notch1 and miR-133a. Molecular Theraphy Nucleic Acids., 21, 636–655. https://doi.org/10.1016/j.omtn.2020.06.024
Wei, M. Y., Lv, R. R., & Teng, Z. (2020). Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. European Review for Medical and Pharmacological Sciences, 24(24), 12849–12858. https://doi.org/10.26355/eurrev_202012_24187
Yang, G., Zhang, Y., & Yang, J. (2019). Identification of potentially functional CircRNA-miRNA-mRNA regulatory network in gastric carcinoma using bioinformatics analysis. Medical Science Monitor, 25, 8777–8796. https://doi.org/10.12659/MSM.916902
Cui, J., Wang, J. S., Wang, S. M., Lian, C., Qiu, J. C., & Liu, Z. (2019). microRNA-106a-5p regulated the funcation human umbilical vein endothelial cells by targeting STAT3. Zhonghua Yi Xue Za Zhi, 99(48), 3814–3818. https://doi.org/10.3760/cma.j.issn.0376-2491.2019.48.011
Kim, J. M., Jung, K. H., Chu, K., Lee, S. T., Ban, J., Moon, J., et al. (2015). Atherosclerosis-related circulating MicroRNAs as a predictor of stroke recurrence. Translational Stroke Research, 6(3), 191–197. https://doi.org/10.1007/s12975-015-0390-1
Xu, L., Hao, H., Hao, Y., Wei, G., Li, G., Ma, P., et al. (2019). Aberrant MFN2 transcription facilitates homocysteine-induced VSMCs proliferation via the increased binding of c-Myc to DNMT1 in atherosclerosis. Journal of Cellular and Molecular Medicine, 23(7), 4611–4626. https://doi.org/10.1111/jcmm.14341
Munoz, J. P., Ivanova, S., Sanchez-Wandelmer, J., Martinez-Cristobal, P., Noguera, E., Sancho, A., et al. (2013). Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO Journal, 32(17), 2348–2361. https://doi.org/10.1038/emboj.2013.168
Boutant, M., Kulkarni, S. S., Joffraud, M., Ratajczak, J., Valera-Alberni, M., Combe, R., et al. (2017). Mfn2 is critical for brown adipose tissue thermogenic function. EMBO Journal, 36(11), 1543–1558. https://doi.org/10.15252/embj.201694914
Feng, S., Gao, L., Zhang, D., Tian, X., Kong, L., Shi, H., et al. (2019). MiR-93 regulates vascular smooth muscle cell proliferation, and neointimal formation through targeting Mfn2. International Journal of Biological Sciences, 15(12), 2615–2626. https://doi.org/10.7150/ijbs.36995
Chen, W. R., Zhou, Y. J., Yang, J. Q., Liu, F., Wu, X. P., & Sha, Y. (2020). Melatonin attenuates calcium deposition from vascular smooth muscle cells by activating mitochondrial fusion and mitophagy via an AMPK/OPA1 signaling pathway. Oxidative Medicine and Cellular Longevity, 2020, 5298483. https://doi.org/10.1155/2020/5298483
Willems, B. A., Furmanik, M., Caron, M. M. J., Chatrou, M. L. L., Kusters, D. H. M., Welting, T. J. M., et al. (2018). Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Science and Reports, 8(1), 4961. https://doi.org/10.1038/s41598-018-23353-y
Perconti, G., Rubino, P., Contino, F., Bivona, S., Bertolazzi, G., Tumminello, M., et al. (2019). RIP-Chip analysis supports different roles for AGO2 and GW182 proteins in recruiting and processing microRNA targets. BMC Bioinformatics, 20(Suppl 4), 120. https://doi.org/10.1186/s12859-019-2683-y
Tesauro, M., Mauriello, A., Rovella, V., Annicchiarico-Petruzzelli, M., Cardillo, C., Melino, G., et al. (2017). Arterial ageing: From endothelial dysfunction to vascular calcification. Journal of Internal Medicine, 281(5), 471–482. https://doi.org/10.1111/joim.12605
Fu, Y., Gao, C., Liang, Y., Wang, M., Huang, Y., Ma, W., et al. (2016). Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circulation Research, 119(2), 261–276. https://doi.org/10.1161/CIRCRESAHA.115.308021
Kuzan, A., Chwilkowska, A., Pezowicz, C., Witkiewicz, W., Gamian, A., Maksymowicz, K., et al. (2017). The content of collagen type II in human arteries is correlated with the stage of atherosclerosis and calcification foci. Cardiovascular Pathology, 28, 21–27. https://doi.org/10.1016/j.carpath.2017.02.003
Morony, S., Tintut, Y., Zhang, Z., Cattley, R. C., Van, G., Dwyer, D., et al. (2008). Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation, 117(3), 411–420. https://doi.org/10.1161/CIRCULATIONAHA.107.707380
Kim, Y. K., & Kook, H. (2019). Diverse roles of noncoding RNAs in vascular calcification. Archives of Pharmacal Research, 42(3), 244–251. https://doi.org/10.1007/s12272-019-01118-z
Ma, C., Gu, R., Wang, X., He, S., Bai, J., Zhang, L., et al. (2020). circRNA CDR1as promotes pulmonary artery smooth muscle cell calcification by upregulating CAMK2D and CNN3 via sponging miR-7-5p. Molecular Theraphy Nucleic Acids., 22, 530–541. https://doi.org/10.1016/j.omtn.2020.09.018
Shen, L., Hu, Y., Lou, J., Yin, S., Wang, W., Wang, Y., et al. (2019). CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Molecular Medicine Reports, 19(5), 3923–3932. https://doi.org/10.3892/mmr.2019.10011
Kang, L., Jia, H., Huang, B., Lu, S., Chen, Z., Shen, J., et al. (2021). Identification of differently expressed mRNAs in atherosclerosis reveals CDK6 is regulated by circHIPK3/miR-637 axis and promotes cell growth in human vascular smooth muscle cells. Frontiers in Genetics, 12, 596169. https://doi.org/10.3389/fgene.2021.596169
Wen, J., Liao, J., Liang, J., Chen, X. P., Zhang, B., & Chu, L. (2020). Circular RNA HIPK3: A key circular RNA in a variety of human cancers. Frontiers in Oncology, 10, 773. https://doi.org/10.3389/fonc.2020.00773
Liu, B., Hou, Q., Ma, Y., & Han, X. (2020). HIPK3 mediates inflammatory cytokines and oxidative stress markers in monocytes in a rat model of sepsis through the JNK/c-Jun signaling pathway. Inflammation, 43(3), 1127–1142. https://doi.org/10.1007/s10753-020-01200-5
Liu, Y., Qian, L., Yang, J., Huang, H., Feng, J., Li, X., et al. (2018). The expression level and prognostic value of HIPK3 among non-small-cell lung cancer patients in China. Oncotargets and Therapy, 11, 7459–7469. https://doi.org/10.2147/OTT.S166878
Panda, A. C. (2018). Circular RNAs act as miRNA sponges. Advances in Experimental Medicine and Biology, 1087, 67–79. https://doi.org/10.1007/978-981-13-1426-1_6
Cuevas, A., Saavedra, N., Cavalcante, M. F., Salazar, L. A., & Abdalla, D. S. (2014). Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Archives of Biochemistry and Biophysics, 557, 28–35. https://doi.org/10.1016/j.abb.2014.04.009
Hu, Y., Xu, R., He, Y., Zhao, Z., Mao, X., Lin, L., et al. (2020). Downregulation of microRNA106a5p alleviates oxLDLmediated endothelial cell injury by targeting STAT3. Molecular Medicine Reports, 22(2), 783–791. https://doi.org/10.3892/mmr.2020.11147
Liu, C., Ge, B., He, C., Zhang, Y., Liu, X., Liu, K., et al. (2014). Mitofusin 2 decreases intracellular lipids in macrophages by regulating peroxisome proliferator-activated receptor-gamma. Biochemical and Biophysical Research Communications, 450(1), 500–506. https://doi.org/10.1016/j.bbrc.2014.06.005
Acknowledgements
This project was supported by Hainan Province Clinical Medical Center
Author information
Authors and Affiliations
Contributions
W. B. Z.: Conceptualization.
Y. F. Q.: Funding acquisition.
Z. X. X.: Writing—original draft.
H. C.: Data curation; resources.
S. H. L.: Methodology; formal analysis.
Z. Z. L.: Investigation; software; visualization.
Z. F. Z.: Project administration; supervision.
H. F. W.: Validation; writing—review and editing.
All authors have read and approved the final version of this manuscript to be published.
Corresponding author
Ethics declarations
Ethical Approval
All participating subjects gave informed consent. This study was approved by the Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Adrian Chester oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

ESM 1
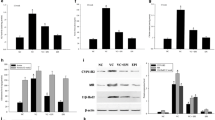
Supplementary Fig. 1 The expression of HIPK3 in atherosclerosis (A) The expression of HIPK3 in normal tissues (n=12) and atherosclerotic tissues (n=9) were detected by RT-qPCR. (B) RT-qPCR detected the expression of HIPK3 in blood samples of patients with atherosclerosis (n=20) and normal volunteers (n=20). *P < 0.05, **P < 0.01, ***P < 0.001. (PNG 26 kb)
Rights and permissions
About this article
Cite this article
Zhang, WB., Qi, YF., **ao, ZX. et al. CircHIPK3 Regulates Vascular Smooth Muscle Cell Calcification Via the miR-106a-5p/MFN2 Axis. J. of Cardiovasc. Trans. Res. 15, 1315–1326 (2022). https://doi.org/10.1007/s12265-022-10247-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10247-8