Abstract
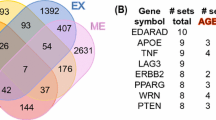
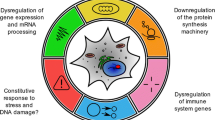
The present review has mainly focused on a systematic investigation of the genes responsible for biological ageing. Ageing has been defined as a successive decline in biological functions, leading to age-associated disorders, which have caused death. Cell homeostasis has been disturbed due to multiple factors such as accumulation of DNA damage, decrease in telomeres, replicative senescence, cell division, metabolism, respiration, autophagy, calorie management, and genetic integrity. This imbalance in cell homeostasis has a major impact on the accelerated biological ageing process. Increased risk of age-associated disorders and mortality rates makes it necessary to know the cellular and molecular mechanisms behind it. This current study provides an overview of genes and their functions associated with dysregulation in core cellular functions such as replication, genetic stability, metabolism, respiration, and autophagy. The genes associated with these biological processes have been identified through a comprehensive literature survey and additional genes were included based on the outcome of STRING analysis. These genes were functionally enriched using gene ontology. Finally, a selected set of genes was mapped with 74 biological functions. Then, a correlation map was plotted to bring out genes with maximum impact on the biological processes involved in ageing. This study not only observed the most commonly known players such as mTOR and SIRT1 but also noticed less-reported genes such as ATM, LRRK2, ERCC1, ATG5, and BECN1 which were also found to be highly impacting the process of biological ageing. Additionally, the gerontology of these top five less-reported genes also has been explored.



Similar content being viewed by others
References
Vik A, Kociński M, Rye I et al (2023) Functional activity level reported by an informant is an early predictor of Alzheimer’s disease. BMC Geriatr 23:205. https://doi.org/10.1186/s12877-023-03849-7
Wadden TA, Bray GA (2018) Handbook of obesity treatment. The Guilford Press, New York
The United Nations and ageing (1999) Nurs J India 90:37–38. https://www.un.org/en/global-issues/ageing
United Nations (2022) World population prospects 2022: summary of results. https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022. Accessed 28 Dec 2022
United Nations Conference on Trade and Development (2022) The least developed countries report 2021: the least developed countries in the post-COVID world–Learning from 50 years of experience. https://unctad.org/board-action/least-developed-countries-report-2021-least-developed-countries-post-covid-world. Accessed 5 Jan 2023
Kaneda T (2006) Health care challenges for develo** countries with ageing populations. https://www.prb.org/resources/health-care-challenges-for-develo**-countries-with-aging-populations/. Accessed 5 June 2023
Flanagan EW, Most J, Mey JT et al (2020) Calorie restriction and aging in humans. Annu Rev Nutr 40:105–133. https://doi.org/10.1146/annurev-nutr-122319-034601
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621. https://doi.org/10.1016/0014-4827(61)90192-6
Effros RB (2007) Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine 25:599–604. https://doi.org/10.1016/j.vaccine.2006.08.032
Schneider EL, Mitsui Y (1976) The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci USA 73:3584–3588. https://doi.org/10.1073/pnas.73.10.3584
Niedernhofer LJ, Robbins PD (2018) Senotherapeutics for healthy ageing. Nat Rev Drug Discov 17:377. https://doi.org/10.1038/nrd.2018.44
Watson JD (1972) Origin of concatemeric T7 DNA. Nat New Biol 239:197–201. https://doi.org/10.1038/newbio239197a0
Li B (2012) Reviews on selected topics of telomere biology. Intechopen, London
Olovnikov AM (1996) Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 31:443–448. https://doi.org/10.1016/0531-5565(96)00005-8
Birch J, Gil J (2020) Senescence and the SASP: many therapeutic avenues. Genes Dev 34:1565–1576. https://doi.org/10.1101/gad.343129.120
Ramos I, Stamatakis K, Oeste CL et al (2020) Vimentin as a multifaceted player and potential therapeutic target in viral infections. Int J Mol Sci 21:4675. https://doi.org/10.3390/ijms21134675
Yang YK, Ogando CR, Wang See C et al (2018) Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther 9:131. https://doi.org/10.1186/s13287-018-0876-3
Kumari R, Jat P (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 9:645593. https://doi.org/10.3389/fcell.2021.645593
Ressler S, Bartkova J, Niederegger H et al (2006) p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5:379–389. https://doi.org/10.1111/j.1474-9726.2006.00231.x
Shin SH, Lee YH, Rho NK et al (2023) Skin aging from mechanisms to interventions: focusing on dermal aging. Front Physiol 14:1195272. https://doi.org/10.3389/fphys.2023.1195272
Stallone G, Infante B, Prisciandaro C et al (2019) mTOR and aging: an old fashioned dress. Int J Mol Sci 20:2774. https://doi.org/10.3390/ijms20112774
Victorelli S, Passos JF (2017) Telomeres and cell senescence—size matters not. EBioMedicine 21:14–20. https://doi.org/10.1016/j.ebiom.2017.03.027
GeneCards (2023) TERT Gene - telomerase reverse transcriptase. https://www.genecards.org/cgi-bin/carddisp.pl?gene=TERT. Accessed 3 June 2023
Bernal A, Tusell L (2018) Telomeres: implications for cancer development. Int J Mol Sci 19:294. https://doi.org/10.3390/ijms19010294
Boutelle AM, Attardi LD (2021) p53 and tumor suppression: it takes a network. Trends Cell Biol 31:298–310. https://doi.org/10.1016/j.tcb.2020.12.011
Zarneshan SN, Fakhri S, Bachtel G et al (2023) Exploiting pivotal mechanisms behind the senescence-like cell cycle arrest in cancer. Adv Protein Chem Struct Biol 135:1–19. https://doi.org/10.1016/bs.apcsb.2022.11.007
Yu A, Dang W (2017) Regulation of stem cell aging by SIRT1—linking metabolic signaling to epigenetic modifications. Mol Cell Endocrinol 455:75–82. https://doi.org/10.1016/j.mce.2017.03.031
De Bonis ML, Ortega S, Blasco MA (2014) SIRT1 is necessary for proficient telomere elongation and genomic stability of induced pluripotent stem cells. Stem Cell Rep 2:690–706. https://doi.org/10.1016/j.stemcr.2014.03.002
Brand MD, Orr AL, Perevoshchikova IV et al (2013) The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol 169(Suppl 2):1–8. https://doi.org/10.1111/bjd.12208
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125:1241–1252. https://doi.org/10.1016/j.cell.2006.06.010
Lima T, Li TY, Mottis A et al (2022) Pleiotropic effects of mitochondria in aging. Nat Aging 2:199–213. https://doi.org/10.1038/s43587-022-00191-2
Yasukawa T, Suzuki T, Ueda T et al (2000) Modification defect at anticodon wobble nucleotide of mitochondrial tRNAs(Leu)(UUR) with pathogenic mutations of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. J Biol Chem 275:4251–4257. https://doi.org/10.1074/jbc.275.6.4251
Hahn A, Zuryn S (2019) Mitochondrial genome (mtDNA) mutations that generate reactive oxygen species. Antioxidants (Basel) 8:392. https://doi.org/10.3390/antiox8090392
Bukowiecki R, Adjaye J, Prigione A (2014) Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology 60:174–182. https://doi.org/10.1159/000355050
Zhang Y, Guo L, Han S et al (2020) Adult mesenchymal stem cell ageing interplays with depressed mitochondrial Ndufs6. Cell Death Dis 11:1075. https://doi.org/10.1038/s41419-020-03289-w
Tavallaie M, Voshtani R, Deng X et al (2020) Moderation of mitochondrial respiration mitigates metabolic syndrome of aging. Proc Natl Acad Sci USA 117:9840–9850. https://doi.org/10.1073/pnas.1917948117
Maiese K (2020) Sirtuin biology in medicine: targeting new avenues of care in development, ageing, and disease. Academic Press, Cambridge
Li P, Liu Y, Burns N et al (2017) SIRT1 is required for mitochondrial biogenesis reprogramming in hypoxic human pulmonary arteriolar smooth muscle cells. Int J Mol Med 39:1127–1136. https://doi.org/10.3892/ijmm.2017.2932
Gureev AP, Shaforostova EA, Popov VN (2019) Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet 10:435. https://doi.org/10.3389/fgene.2019.00435
Copeland WC (2010) The mitochondrial DNA polymerase in health and disease. Subcell Biochem 50:211–222. https://doi.org/10.1007/978-90-481-3471-7_11
Rahman S, Copeland WC (2019) POLG-related disorders and their neurological manifestations. Nat Rev Neurol 15:40–52. https://doi.org/10.1038/s41582-018-0101-0
Kitagishi Y, Nakano N, Ogino M et al (2017) PINK1 signaling in mitochondrial homeostasis and in aging (Review). Int J Mol Med 39:3–8. https://doi.org/10.3892/ijmm.2016.2827
Crabbe L, Jauch A, Naeger CM et al (2007) Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc Natl Acad Sci USA 104:2205–2210. https://doi.org/10.1073/pnas.0609410104
Duesberg P, Fabarius A, Hehlmann R (2004) Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life 56:65–81. https://doi.org/10.1080/15216540410001667902
Sun Z, Zhao C, Liu X et al (2023) Mutation analysis of the ECE1 gene in late-onset Alzheimer’s disease. Neurobiol Aging 129:58–61. https://doi.org/10.1016/j.neurobiolaging.2023.05.002
Salk D, Fujiwara Y, Martin GM (2013) Werner’s syndrome and human ageing. Springer, New York
Shen JC, Gray MD, Oshima J et al (1998) Werner syndrome protein. I. DNA helicase and dna exonuclease reside on the same polypeptide. J Biol Chem 273:34139–34144. https://doi.org/10.1074/jbc.273.51.34139
Lebel M (2004) Molecular mechanisms of Werner’s syndrome. Springer, New York
Yamagata K, Kato J, Shimamoto A et al (1998) Bloom’s and Werner’s syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases. Proc Natl Acad Sci USA 95:8733–8738. https://doi.org/10.1073/pnas.95.15.8733
Wazir U, Ahmed MH, Bridger JM et al (2013) The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett 18:595–611. https://doi.org/10.2478/s11658-013-0109-9
Jones MK, Gobert GN, Zhang L et al (2004) The cytoskeleton and motor proteins of human schistosomes and their roles in surface maintenance and host-parasite interactions. BioEssays 26:752–765. https://doi.org/10.1002/bies.20058
Piekarowicz K, Machowska M, Dzianisava V et al (2019) Hutchinson-Gilford progeria syndrome-current status and prospects for gene therapy treatment. Cells 8:88. https://doi.org/10.3390/cells8020088
Sriramulu S, Thoidingjam S, Brown SL et al (2023) Molecular targets that sensitize cancer to radiation killing: from the bench to the bedside. Biomed Pharmacother 158:114126. https://doi.org/10.1016/j.biopha.2022.114126
Bergom C, West CM, Higginson DS et al (2019) The implications of genetic testing on radiation therapy decisions: a guide for radiation oncologists. Int J Radiat Oncol Biol Phys 105:698–712. https://doi.org/10.1016/j.ijrobp.2019.07.026
Nam EA (2011) Phospho-regulation of the DNA damage response kinase ATR. Dissertation, Vanderbilt University
Manandhar M, Boulware KS, Wood RD (2015) The ERCC1 and ERCC4 (XPF) genes and gene products. Gene 569:153–161. https://doi.org/10.1016/j.gene.2015.06.026
Rapin I, Lindenbaum Y, Dickson DW et al (2000) Cockayne syndrome and xeroderma pigmentosum. Neurology 55:1442–1449. https://doi.org/10.1212/wnl.55.10.1442
Revuelta M, Matheu A (2017) Autophagy in stem cell aging. Aging Cell 16:912–915. https://doi.org/10.1111/acel.12655
Chang NC (2020) Autophagy and stem cells: self-eating for self-renewal. Front Cell Dev Biol 8:138. https://doi.org/10.3389/fcell.2020.00138
Phadwal K, Watson AS, Simon AK (2013) Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cell Mol Life Sci 70:89–103. https://doi.org/10.1007/s00018-012-1032-3
Rastaldo R, Vitale E, Giachino C (2020) Dual role of autophagy in regulation of mesenchymal stem cell senescence. Front Cell Dev Biol 8:276. https://doi.org/10.3389/fcell.2020.00276
Kondrikov D, Elmansi A, Bragg RT et al (2020) Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol 130:110805. https://doi.org/10.1016/j.exger.2019.110805
Wan Y, Zhuo N, Li Y et al (2017) Autophagy promotes osteogenic differentiation of human bone marrow mesenchymal stem cell derived from osteoporotic vertebrae. Biochem Biophys Res Commun 488:46–52. https://doi.org/10.1016/j.bbrc.2017.05.004
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) mTOR is a key modulator of ageing and age-related disease. Nature 493:338–345. https://doi.org/10.1038/nature11861
Cuervo AM, Dice JF (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273:501–503. https://doi.org/10.1126/science.273.5274.501
Cuervo AM, Dice JF (2000) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113(Pt 24):4441–4450. https://doi.org/10.1242/jcs.113.24.4441
Hui KY, Fernandez-Hernandez H, Hu J et al (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med 10:eaai7795. https://doi.org/10.1126/scitranslmed.aai7795
Plowey ED, Cherra SJ 3rd, Liu YJ et al (2008) Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem 105:1048–1056. https://doi.org/10.1111/j.1471-4159.2008.05217.x
Saha S, Ash PE, Gowda V et al (2015) Mutations in LRRK2 potentiate age-related impairment of autophagic flux. Mol Neurodegener 10:26. https://doi.org/10.1186/s13024-015-0022-y
Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell 146:682–695. https://doi.org/10.1016/j.cell.2011.07.030
Park JM, Jung CH, Seo M et al (2016) The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 12:547–564. https://doi.org/10.1080/15548627.2016.1140293
Manifava M, Ktistakis NT (2021) Autophagy on the road to longevity and ageing. In: Rothermel BA, Diwan A (eds) Autophagy in health and disease, 2nd edn. Elsevier, San Diego
Parker D, Doraiswamy PM, Kraus W et al (2022) Impact of calorie restriction on plasma Alzheimer’s disease biomarkers in healthy young and middle-aged adults. Innov Aging 6:97–98. https://doi.org/10.1093/geroni/igac059.388
Ahima RS (2009) Connecting obesity, aging and diabetes. Nat Med 15:996–997. https://doi.org/10.1038/nm0909-996
Ritov VB, Menshikova EV, He J et al (2005) Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54:8–14. https://doi.org/10.2337/diabetes.54.1.8
Al-Aubaidy HA, Jelinek HF (2011) Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol 164:899–904. https://doi.org/10.1530/EJE-11-0053
Ahn KS, Aggarwal BB (2005) Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci 1056:218–233. https://doi.org/10.1196/annals.1352.026
Chung HY, Cesari M, Anton S et al (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30. https://doi.org/10.1016/j.arr.2008.07.002
Yang SR, Wright J, Bauter M et al (2007) Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292:L567–L576. https://doi.org/10.1152/ajplung.00308.2006
Wahli W, Guillou H (2021) Metabolism and metabolomics of liver in health and disease. MDPI, Basel
Corton JC, Brown-Borg HM (2005) Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci 60:1494–1509. https://doi.org/10.1093/gerona/60.12.1494
Villanueva-Paz M, Cotán D, Garrido-Maraver J et al (2016) AMPK regulation of cell growth, apoptosis, autophagy, and bioenergetics. Exp Suppl 107:45–71. https://doi.org/10.1007/978-3-319-43589-3_3
Salašová A, Yokota C, Potěšil D et al (2017) A proteomic analysis of LRRK2 binding partners reveals interactions with multiple signaling components of the WNT/PCP pathway. Mol Neurodegener 12:54. https://doi.org/10.1186/s13024-017-0193-9
Harvey K, Outeiro TF (2019) The role of LRRK2 in cell signalling. Biochem Soc Trans 47:197–207. https://doi.org/10.1042/BST20180464
Berwick DC, Harvey K (2012) LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6. Hum Mol Genet 21:4966–4979. https://doi.org/10.1093/hmg/dds342
Feng ST, Wang ZZ, Yuan YH et al (2020) Dynamin-related protein 1: a protein critical for mitochondrial fission, mitophagy, and neuronal death in Parkinson’s disease. Pharmacol Res 151:104553. https://doi.org/10.1016/j.phrs.2019.104553
Phan LM, Rezaeian AH (2021) ATM: main features, signaling pathways, and its diverse roles in DNA damage response, tumor suppression, and cancer development. Genes (Basel) 12:845. https://doi.org/10.3390/genes12060845
Maréchal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5:a012716. https://doi.org/10.1101/cshperspect.a012716
Nowsheen S, Yang ES (2012) The intersection between DNA damage response and cell death pathways. Exp Oncol 34:243–254
Ambrose M, Gatti RA (2013) Pathogenesis of ataxia-telangiectasia: the next generation of ATM functions. Blood 121:4036–4045. https://doi.org/10.1182/blood-2012-09-456897
Lee SS, Bohrson C, Pike AM et al (2015) ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep 13:1623–1632. https://doi.org/10.1016/j.celrep.2015.10.035
Qian M, Liu Z, Peng L et al (2018) Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria. Elife 7:e34836. https://doi.org/10.7554/eLife.34836
Schärer OD (2013) Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol 5:a012609. https://doi.org/10.1101/cshperspect.a012609
Kim DE, Dollé MET, Vermeij WP et al (2020) Deficiency in the DNA repair protein ERCC1 triggers a link between senescence and apoptosis in human fibroblasts and mouse skin. Aging Cell 19:e13072. https://doi.org/10.1111/acel.13072
Aman Y, Schmauck-Medina T, Hansen M et al (2021) Autophagy in healthy aging and disease. Nat Aging 1:634–650. https://doi.org/10.1038/s43587-021-00098-4
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460–473. https://doi.org/10.1089/ars.2013.5371
Hale AN, Ledbetter DJ, Gawriluk TR et al (2013) Autophagy: regulation and role in development. Autophagy 9:951–972. https://doi.org/10.4161/auto.24273
Son JH, Shim JH, Kim KH et al (2012) Neuronal autophagy and neurodegenerative diseases. Exp Mol Med 44:89–98. https://doi.org/10.3858/emm.2012.44.2.031
Kang R, Zeh HJ, Lotze MT et al (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18:571–580. https://doi.org/10.1038/cdd.2010.191
Yim WW, Mizushima N (2020) Lysosome biology in autophagy. Cell Discov 6:6. https://doi.org/10.1038/s41421-020-0141-7
Acknowledgements
I would like to acknowledge the Drug Testing Laboratory, Saveetha Institute of Medical and Technical Sciences, for providing the facility for completing my computational work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desai, S.C., Macrin, A.D., Senthilvelan, T. et al. Identification of genes associated with accelerated biological ageing through computational analysis: a systematic review. Biotechnol Bioproc E (2024). https://doi.org/10.1007/s12257-024-00113-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12257-024-00113-6