Abstract
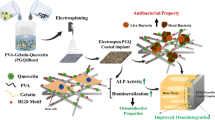
This research study deals with the development of copper nanoparticles (CN) and nano-hydroxyapatite (nHAP) infused chitosan (C) and gelatin (G) based nanocomposite scaffolds for bone tissue engineering applications. Human-origin osteoblast cells (MG-63) were seeded over the scaffolds to investigate the novel biomimetic extracellular matrix system. The scanning electron microscopy (SEM) showed an average pore size between 100–146 µm for all the C-G-nHAP-CN based scaffolds. The in-vitro degradation study showed 74–83% degradation for CN-based scaffolds. For 0.03% CN based scaffold degradation rate (84%) was very close to the control scaffold. Swelling ratio was highest for the chitosan scaffold and it was in the range between 5.25–5.93 mg/mL for other scaffolds. Compressive moduli were highest for 0.03% CN scaffold (3.32 MPa) which was relatively very high in comparison to C-G-nHAP scaffold with 2.4 MPa strength in a wet state. Stress-strain graphs also show the maximum displacement by 0.03% CN scaffold. The functional and structural analysis for the scaffolds showed the presence of nHAP in the scaffold and CN peaks within the composite structure. Differential scanning colorimetry testing showed reduced crystallinity in CN-based scaffolds with a melting temperature of 320°C. Their 2D cell behaviour in the Electrical Cell Impedance System (ECIS) study showed maximum cell spreading and growth in 0.02% CN-based scaffold. The cell-seeded composite was tested for 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), 4,6-diamidino-2-phenylindole (DAPI), and acridine orange and propidium iodide (AOPI) assay for testing its cytocompatibility for MG-63 cell line. Cell proliferation and cell spreading was observed by SEM in all the CN-based scaffolds. Alkaline phosphatase (ALP) activity was highest in 0.03% CN scaffold with 2.0 optical density (OD) value. Alizarin Red Stain (ARS) staining was performed to support this study. It can be statistically depicted that nHAP and 0.03% CN-based scaffold could be potential biomaterial for minor to severe bone-related tissue regeneration applications.
Similar content being viewed by others
References
Fernandez de Grado, G., L. Keller, Y. Idoux-Gillet, Q. Wagner, A.-M. Musset, N. Benkirane-Jessel, F. Bornert, and D. Offner (2018) Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 9: 2041731418776819.
Amini, A. R., C. T. Laurencin, and S. P. Nukavarapu (2012) Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 40: 363–408.
Oryan, A., S. Alidadi, A. Moshiri, and N. Maffulli (2014) Bone regenerative medicine: classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 9: 18.
Sheikh, Z., S. Najeeb, Z. Khurshid, V. Verma, H. Rashid, and M. Glogauer (2015) Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel) 8: 5744–5794.
Feng, X. (2009) Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr. Chem. Biol. 3: 189–196.
Nikolova, M. P. and M. S. Chavali (2019) Recent advances in biomaterials for 3D scaffolds: a review. Bioact. Mater. 4: 271–292.
Grass, G., C. Rensing, and M. Solioz (2011) Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 77: 1541–1547.
**e, H. and Y. J. Kang (2009) Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 16: 1304–1314.
D’Andrea, L. D., A. Romanelli, R. Di Stasi, and C. Pedone (2010) Bioinorganic aspects of angiogenesis. Dalton Trans. 39: 7625–7636.
Turski, M. L. and D. J. Thiele (2009) New roles for copper metabolism in cell proliferation, signaling, and disease. J. Biol. Chem. 284: 717–721.
Kumar, N. and L. S. B. Upadhyay (2020) Enzyme immobilization over polystyrene surface using cysteine functionalized copper nanoparticle as a linker molecule. Appl. Biochem. Biotechnol. 191: 1247–1257.
Zhou, M., M. Tian, and C. Li (2016) Copper-based nanomaterials for cancer imaging and therapy. Bioconjug. Chem. 27: 1188–1199.
Halevas, E. G. and A. A. Pantazaki (2018) Copper nanoparticles as therapeutic anticancer agents. Nanomed. Nanotechnol. J. 2: 119–139.
**, R., C. Hiemstra, Z. Zhong, and J. Feijen (2007) Enzymemediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials 28: 2791–2800.
Kudr, J., Y. Haddad, L. Richtera, Z. Heger, M. Cernak, V. Adam, and O. Zitka (2017) Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials (Basel) 7: 243.
Kumari, S., B. N. Singh, and P. Srivastava (2019) Effect of copper nanoparticles on physico-chemical properties of chitosan and gelatin-based scaffold developed for skin tissue engineering application. 3 Biotech 9: 102.
Maji, K. and S. Dasgupta (2014) Hydroxyapatite-chitosan and gelatin based scaffold for bone tissue engineering. Trans. Indian Ceram. Soc. 73: 110–114.
Echave, M. C., L. Saenz del Burgo, J. L. Pedraz, and G. Orive (2017) Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 23: 3567–3584.
Raucci, M. G., U. D’Amora, A. Ronca, C. Demitri, and L. Ambrosio (2019) Bioactivation routes of gelatin-based scaffolds to enhance at nanoscale level bone tissue regeneration. Front. Bioeng. Biotechnol. 7: 27.
Elieh-Ali-Komi, D. and M. R. Hamblin (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. (Indore) 4: 411–427.
Zang, S., L. Zhu, K. Luo, R. Mu, F. Chen, X. Wei, X. Yan, B. Han, X. Shi, Q. Wang, and L. ** (2017) Chitosan composite scaffold combined with bone marrow-derived mesenchymal stem cells for bone regeneration: in vitro and in vivo evaluation. Oncotarget 8: 110890–110903.
Tang, T., G. Zhang, C. P. Y. Lau, L. Z. Zheng, X. H. **e, X. L. Wang, X. H. Wang, K. He, Y. Patrick, L. Qin, and S. M. Kumta (2011) Effect of water-soluble P-chitosan and S-chitosan on human primary osteoblasts and giant cell tumor of bone stromal cells. Biomed. Mater. 6: 015004.
Gadgey, K. K. and G. S. Sharma (2020) Investigation of mechanical properties of chitosan based films prepared from Narmada riverside crab shells. Int. J. Mech. Eng. Technol. 11: 21–28.
Maji, K., S. Dasgupta, B. Kundu, and A. Bissoyi (2015) Development of gelatin-chitosan-hydroxyapatite based bioactive bone scaffold with controlled pore size and mechanical strength. J. Biomater. Sci. Polym. Ed. 26: 1190–1209.
Wang, Y., W. Zhang, and Q. Yao (2021) Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Translat. 29: 60–71.
Maia, F. R., V. M. Correlo, J. M. Oliveira, and R. L. Reis (2019) Natural origin materials for bone tissue engineering: properties, processing, and performance. pp. 535–558. In: A. Atala, R. Lanza, A. G. Mikos, and R. Nerem (eds.). Principles of Regenerative Medicine. 3rd ed. Academic Press, London, UK.
Polo-Corrales, L., M. Latorre-Esteves, and J. E. Ramirez-Vick (2014) Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 14: 15–56.
Kattimani, V. S., S. Kondaka, and K. P. Lingamaneni (2016) Hydroxyapatite-past, present, and future in bone regeneration. Bone Tissue Regen. Insights 7: BTRI.S36138.
Tampieri, A., M. Iafisco, S. Sprio, A. Ruffini, S. Panseri, M. Montesi, A. Adamiano, and M. Sandri (2016) Hydroxyapatite: from nanocrystals to hybrid nanocomposites for regenerative medicine. pp. 119–144. In: I. V. Antoniac (ed.). Handbook of Bioceramics and Biocomposites. Springer, Cham, Switzerland.
Turnbull, G., J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li, and W. Shu (2018) 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 3: 278–314.
Dan, Y., O. Liu, Y. Liu, Y.-Y. Zhang, S. Li, X. Feng, Z. Shao, C. Yang, S.-H. Yang, and J. Hong (2016) Development of novel biocomposite scaffold of chitosan-gelatin/nanohydroxyapatite for potential bone tissue engineering applications. Nanoscale Res. Lett. 11: 487.
Guan, J., K. L. Fujimoto, M. S. Sacks, and W. R. Wagner (2005) Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 26: 3961–3971.
Basu, P., N. Saha, and P. Saha (2020) Swelling and rheological study of calcium phosphate filled bacterial cellulose-based hydrogel scaffold. J. Appl. Polym. Sci. 137: 48522.
Wang, P.-Y. and W.-B. Tsai (2013) Modulation of the proliferation and matrix synthesis of chondrocytes by dynamic compression on genipin-crosslinked chitosan/collagen scaffolds. J. Biomater. Sci. Polym. Ed. 24: 507–519.
Singh, B. N., V. Veeresh, S. P. Mallick, S. Sinha, A. Rastogi, and P. Srivastava (2020) Generation of scaffold incorporated with nanobioglass encapsulated in chitosan/chondroitin sulfate complex for bone tissue engineering. Int. J. Biol. Macromol. 153: 1–16.
Sahi, A. K., N. Varshney, S. Poddar, S. Gundu, and S. K. Mahto (2021) Fabrication and characterization of silk fibroin-based nanofibrous scaffolds supplemented with gelatin for corneal tissue engineering. Cells Tissues Organs 210: 173–194.
Jain, M., R. G. Vaze, S. C. Ugrani, and K. P. Sharma (2018) Mechanoresponsive and recyclable biocatalytic sponges from enzyme-polymer surfactant conjugates and nanoparticles. RSC Adv. 8: 39029–39038.
Zernik, J., K. Twarog, and W. B. Upholt (1990) Regulation of alkaline phosphatase and alpha 2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation 44: 207–215.
Chandrasekar, A., S. Sagadevan, and A. Dakshnamoorthy (2013) Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 8: 1639–1645.
Mokhtari, A., H. Belhouchet, and A. Guermat (2019) In situ high-temperature X-ray diffraction, FT-IR and thermal analysis studies of the reaction between natural hydroxyapatite and aluminum powder. J. Therm. Anal. Calorim. 136: 1515–1526.
Abbasi, N., S. Hamlet, R. M. Love, and N.-T. Nguyen (2020) Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 5: 1–9.
Pradini, D., H. Juwono, K. A. Madurani, and F. Kurniawan (2018) A preliminary study of identification halal gelatin using quartz crystal microbalance (QCM) sensor. Malays. J. Fundam. Appl. Sci. 14: 325–330.
Lewandowska, K., A. Sionkowska, S. Grabska, and M. Michalska (2017) Characterisation of chitosan/hyaluronic acid blend films modified by collagen. Prog. Chem. Appl. Chitin Deriv. 22: 125–134.
Konovalova, I., V. Novikov, Y. Kuchina, and N. Dolgopiatova (2020) Technology and properties of chondroitin sulfate from marine hydrobionts. KnE Life Sci. 5: 305–314.
Isikli, C., V. Hasirci, and N. Hasirci (2012) Development of porous chitosan-gelatin/hydroxyapatite composite scaffolds for hard tissue-engineering applications. J. Tissue Eng. Regen. Med. 6: 135–143.
Sheikh, L., S. Sinha, Y. N. Singhababu, V. Verma, S. Tripathy, and S. Nayar (2018) Traversing the profile of biomimetically nanoengineered iron substituted hydroxyapatite: synthesis, characterization, property evaluation, and drug release modeling. RSC Adv. 8: 19389–19401.
Kumar, P., B. S. Dehiya, and A. Sindhu (2017) Comparative study of chitosan and chitosan-gelatin scaffold for tissue engineering. Int. Nano Lett. 7: 285–290.
Lim, J. Y., J. C. Hansen, C. A. Siedlecki, J. Runt, and H. J. Donahue (2005) Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2: 97–108.
Lee, S. J., J. S. Choi, K. S. Park, G. Khang, Y. M. Lee, and H. B. Lee (2004) Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes. Biomaterials 25: 4699–4707.
Qu, F., J. L. Holloway, J. L. Esterhai, J. A. Burdick, and R. L. Mauck (2017) Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat. Commun. 8: 1780.
Singh, B. N., V. Veeresh, S. P. Mallick, Y. Jain, S. Sinha, A. Rastogi, and P. Srivastava (2019) Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 133: 817–830.
Peter, M., N. Ganesh, N. Selvamurugan, S. V. Nair, T. Furuike, H. Tamura, and R. Jayakumar (2010) Preparation and characterization of chitosan-gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 80: 687–694.
Shuai, C., W. Yang, C. He, S. Peng, C. Gao, Y. Yang, F. Qi, and P. Feng (2020) A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 185: 108275.
Alsberg, E., H. J. Kong, Y. Hirano, M. K. Smith, A. Albeiruti, and D. J. Mooney (2003) Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 82: 903–908.
Fu, Q., M. N. Rahaman, H. Fu, and X. Liu (2010) Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. A 95: 164–171.
Siddiquei, H. R., A. N. Nordin, M. I. Ibrahimy, M. A. Arifin, N. H. Sulong, M. Mel, and I. Voiculescu (2010) Electrical cellsubstrate impedance sensing (ECIS) based biosensor for characterization of DF-1 cells. Proceedings of International Conference on Computer and Communication Engineering (ICCCE’10). May 11–12. Kuala Lumpur, Malaysia.
Kashiwazaki, H., Y. Kishiya, A. Matsuda, K. Yamaguchi, T. Iizuka, J. Tanaka, and N. Inoue (2009) Fabrication of porous chitosan/hydroxyapatite nanocomposites: their mechanical and biological properties. Biomed. Mater. Eng. 19: 133–140.
Pepla, E., L. K. Besharat, G. Palaia, G. Tenore, and G. Migliau (2014) Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann. Stomatol. (Roma) 5: 108–114.
Acknowledgements
Central Instrument Facility Center, IIT (BHU) Varanasi supported this research study by providing several instruments for it. It was funded by SPARC, IUSSTF also. We are also thankful to Dr. Li for providing his lab to perform ECIS experiments. The authors are grateful to the Ministry of Human Resource Development for granting the fellowship for completing our research work. Finally IIT (BHU) for all the lab facilities provided for carrying out the research work to the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare there is no conflict of interest. Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, S., Mishra, A., Singh, D. et al. In-vitro Studies on Copper Nanoparticles and Nano-hydroxyapatite Infused Biopolymeric Composite Scaffolds for Bone Bioengineering Applications. Biotechnol Bioproc E 28, 162–180 (2023). https://doi.org/10.1007/s12257-022-0236-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-022-0236-0