Abstract
Over the past few decades, photocatalysis technology has received extensive attention because of its potential to mitigate or solve energy and environmental pollution problems.Designing novel materials with outstanding photocatalytic activities has become a research hotspot in this field. In this study, we prepared a series of photocatalysts in which BiOCl nanosheets were modified with carbon quantum dots (CQDs) to form CQDs/BiOCl composites by using a simple solvothermal method. The photocatalytic performance of the resulting CQDs/BiOCl composite photocatalysts was assessed by rhodamine B and tetracycline degradation under visible-light irradiation. Compared with bare BiOCl, the photocatalytic activity of the CQDs/BiOCl composites was significantly enhanced, and the 5 wt% CQDs/BiOCl composite exhibited the highest photocatalytic activity with a degradation efficiency of 94.5% after 30 min of irradiation. Moreover, photocatalytic N2 reduction performance was significantly improved after introducing CQDs. The 5 wt% CQDs/BiOCl composite displayed the highest photocatalytic N2 reduction performance to yield NH3 (346.25 μmol/(g h)), which is significantly higher than those of 3 wt% CQDs/BiOCl (256.04 μmol/(g h)), 7 wt% CQDs/BiOCl (254.07 μmol/(g h)), and bare BiOCl (240.19 μmol/(g h)). Our systematic characterizations revealed that the key role of CQDs in improving photocatalytic performance is due to their increased light harvesting capacity, remarkable electron transfer ability, and higher photocatalytic activity sites.
Graphical Abstract
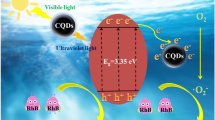
This work reports a novel CQDs/BiOCl composite photocatalyst for efficiently removing contaminants from water.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, ultrathin two-dimensional (2D) materials have garnered immense attention due to their excellent chemical and physical properties [1,13]. When the same semiconductors are reduced to ultralow thickness, photogenerated carriers can be quickly transferred from the inside to the surface, thus resulting in faster charge carrier transfer. Moreover, ultrathin structure materials expose more catalytically active sites, which facilitate surface reactions [12]. Thus, constructing ultrathin 2D materials with suitable band gaps is a desirable approach for designing efficient photocatalysts.
Bismuth oxychloride (BiOCl), as a layered material with good prospects for photocatalytic energy conversion and environmental remediation, has attracted extensive attention in recent years [14,13, 27, 28]. Among them, the synthesis of ultrathin nanosheets is an effective approach. According to the formula t = d2/k2D (where d, k, and D are the particle size, constant, and diffusion coefficient, respectively) [29], the ultrathin thickness of BiOCl allows a reduced d value, whereas self-built internal electric fields lead to an increased D value [2g–i).
The photocatalytic performance of the obtained samples was then assessed for RhB degradation under visible-light irradiation. Before the photocatalytic degradation, a blank experiment was conducted (Fig. S4). From Fig. 3a, 80.6% of RhB could be removed using bare BiOCl material after 30 min of irradiation. With the introduction of CQDs to BiOCl, the photocatalytic performance of BiOCl was significantly improved. The 5 wt% CQDs/BiOCl composite exhibited the highest photocatalytic performance with a degradation efficiency of 94.5% after 30 min of irradiation. However, a further increase in the CQD content beyond 5 wt% resulted in a decrease in photocatalytic performance. Although the modification of BiOCl with CQDs can facilitate charge transfer, excessive CQDs covering the BiOCl surface may limit light absorption [54]. As shown in Fig. 4g, the superoxide radical (·O2−) was observed for the as-prepared materials under visible-light irradiation. The ·O2− intensity of the CQDs/BiOCl composite was significantly higher than that of bare BiOCl (Fig. 4g). As the generation of ·O2− originates from O2 reduction through one-electron transfer, the higher ·O2− intensity of the CQDs/BiOCl composites confirms that CQDs allow more photogenerated electrons to reduce O2. In fact, the delocalized conjugated structure of CQDs allows them to transfer photogenerated electrons easily [55]. From Fig. 4h, the hydroxyl radical (·OH) was also detected under visible-light irradiation, and the generated amounts of ·OH were increased with increasing CQD content. Moreover, Fig. 4i displays the singlet oxygen spectra of the as-prepared samples under UV irradiation. The generated amounts of singlet oxygen by CQDs/BiOCl were increased with increasing in CQD content, which is beneficial for the removal of pollutants. Catalytic measurements have revealed that the CQDs/BiOCl material can be utilized as an efficient photocatalytic degradation system. However, it is still unclear how the photocatalytic process works. To decode the mechanism, a series of control experiments were conducted to analyze the process (Fig. S11). Only 8.7% and 10.5% of TC degradation occurred in the absence of the catalyst and light, respectively, indicating that light and catalyst are indeed required for the reaction. When the reaction was conducted under an argon atmosphere, 56.3% of TC was degraded, which should be driven by photogenerated holes. To determine the types of reactive oxygen species responsible for photocatalytic degradation in our system, we performed characterization using different scavengers, confirming that photogenerated holes are indispensable for the photocatalytic degradation of TC. The control experiment also showed that the reaction activity is largely suppressed using Na2S as a hole scavenger. Moreover, ·O2− was confirmed to play a significant role in the photocatalytic oxidation reaction by ESR analysis (Fig. 4g) and free radical trap** experiments (Fig. S11).
Moreover, the valence band (VB) and conduction band (CB) potentials were calculated using ECB = EVB − Eg, where EVB is the VB edge potential, ECB is the CB edge potential, and Eg is the band gap energy. As observed in Fig. S12, the Eg value of 5 wt% CQDs/BiOCl was 2.92 eV. Figure S13 shows that the EVB value of 5 wt% CQDs/BiOCl was 1.21 eV. From ECB = EVB − Eg, the ECB value of 5 wt% CQDs/BiOCl was calculated to be − 1.71 eV. Here, electrons with energy above ECB (− 1.71 eV) can reduce O2 to generate ·O2− because E0(O2/·O2−) is − 0.046 eV (vs. NHE) [56, 57]. From the above discussion and results, the mechanism of organic pollutant degradation was proposed, as illustrated in Fig. S14. CQDs with remarkable electrical conductivity were introduced into the BiOCl materials as charge mediators. Due to the bridge effect between the two substances, the separation efficiency of the change carriers was significantly enhanced, which offers more electrons to generate the ·O2− active species. Moreover, CQDs can also absorb light at longer wavelengths than BiOCl [58], extending the range of light absorption. Thus, the photocatalytic activity of CQDs/BiOCl was significantly improved after the introduction of CQDs.
Conclusions
In this work, a novel CQDs/BiOCl composite photocatalyst was prepared by using a facile hydrothermal method. The CQDs were integrated on the surface of the BiOCl ultrathin nanosheets to form a tight junction. After introducing CQDs, the photocatalytic activity of the CQDs/BiOCl composites in RhB and TC degradation was significantly enhanced under visible-light irradiation. The 5 wt% CQDs/BiOCl material exhibited the highest photocatalytic performance with a degradation efficiency of 94.5% after 30 min of irradiation. Moreover, the N2 photoreduction performance was significantly improved after introducing CQDs. The 5 wt% CQDs/BiOCl demonstrated a nitrogen photoreduction performance to yield NH3 of 346.25 μmol/(g h), which is significantly higher than those of 3 wt% CQDs/BiOCl (256.04 μmol/(g h)), 7 wt% CQDs/BiOCl (254.07 μmol/(g h)), and bare BiOCl (240.19 μmol/(g h)). The key role of CQDs in improving photocatalytic performance was ascribed to their increased light harvesting capacity, outstanding electron transfer ability, and higher photocatalytic active sites. From the ESR and free radical trap** analysis results, holes and ·O2− were the main active species. This study provides insights into the design of composite photocatalysts by integrating 2D materials and quantum dots.
References
Choi SH, Yun SJ, Won YS et al (2022) Large-scale synthesis of graphene and other 2D materials towards industrialization. Nat Commun 13(1):1484
Li L, **a Y, Zeng M et al (2022) Facet engineering of ultrathin two-dimensional materials. Chem Soc Rev 51(17):7327–7343
Zhou Y, Xu L, Liu M et al (2022) Viscous solvent-assisted planetary ball milling for the scalable production of large ultrathin two-dimensional materials. ACS Nano 16(7):10179–10187
Peng J, Dong W, Wang Z et al (2020) Recent advances in 2D transition metal compounds for electrocatalytic full water splitting in neutral media. Mater Today Adv 8:100081
Di J, Chen C, Zhu C et al (2019) Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. ACS Appl Mater Interfaces 11(34):30786–30792
Song P, Wang M, Di J et al (2020) Reusable graphitic carbon nitride nanosheet-based aerogels as sorbents for oils and organic solvents. ACS Appl Nano Mater 3(8):8176–8181
Sun Y, Gao S, **e Y (2014) Atomically-thick two-dimensional crystals: electronic structure regulation and energy device construction. Chem Soc Rev 43(2):530–546
Zhou Y, Song P, Pan M et al (2023) Super hydrophilic-electrons acceptor regulated rutile TiO2 nanorods for promoting photocatalytic H2 evolution. Appl Surf Sci 623:157098
Zhang J, Song X, Wang L et al (2021) Ultrathin two-dimensional hybrid perovskites toward flexible electronics and optoelectronics. Natl Sci Rev 9(5):nwab129
Shao T, Wang X, Dong H et al (2022) A stacked plasmonic metamaterial with strong localized electric field enables highly efficient broadband light-driven CO2 hydrogenation. Adv Mater 34(28):e2202367
Song P, Di J, Chen H et al (2020) A three-dimensional porous MoS2–PVP aerogel as a highly efficient and recyclable sorbent for oils and organic solvents. Mater Adv 1(4):760–766
Sun Y, Sun Z, Gao S et al (2012) Fabrication of flexible and freestanding zinc chalcogenide single layers. Nat Commun 3:1057
Zhang JJ, Di J, Zhao YP et al (2023) Synergistic defect and do** engineering building strong bonded S-scheme heterojunction for photocatalysis. Chemosphere 344:140347
Liang H, Song P, Jiang W et al (2023) Indium-based atomic layer for photoreduction reactions: design, synthesis and performance optimization. Sep Purif Technol 324:124514
**ong J, Song P, Di J et al (2020) Atomic-level active sites steering in ultrathin photocatalysts to trigger high efficiency nitrogen fixation. Chem Eng J 402:126208
Di J, Chen C, Zhu C et al (2021) Cobalt nitride as a novel cocatalyst to boost photocatalytic CO2 reduction. Nano Energy 79:105429
Yao L, Yang H, Chen Z et al (2020) Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 273:128576
Dong Y, Pang S, Zhang F et al (2023) A novel lateral epitaxial Bi2O3@BiOCl heterostructure for photocatalytic antibiotic degradation in an internal circulation fluidized bed reactor. Chem Eng J 478:147540
Li Q, Ren J, Hao YJ et al (2022) Insight into reactive species-dependent photocatalytic toluene mineralization and deactivation pathways via modifying hydroxyl groups and oxygen vacancies on BiOCl. Appl Catal B Environ 317:121761
Song P, Du J, Ma X et al (2023) Design of Bi4O5Br2/g-C3N4 heterojunction for efficient photocatalytic removal of persistent organic pollutants from water. EcoEnergy 1(1):197–206
Zhao K, Zhang L, Wang J et al (2013) Surface structure-dependent molecular oxygen activation of BiOCl single-crystalline nanosheets. J Am Chem Soc 135(42):15750–15753
Li ZQ, Chen XH, Li T et al (2023) Crystal facet engineering of polar single crystal BiOCl with improved piezo-photocatalytic activity. Appl Surf Sci 615:156283
Cao D, Xu W, Chen S et al (2023) Visualizing catalytic dynamics processes via synchrotron radiation multitechniques. Adv Mater 35(30):e2205346
Liu C, Ren Y, Wang Z et al (2022) Flowerlike BiOCl nanospheres fabricated by an in situ self-assembly strategy for efficiently enhancing photocatalysis. J Colloid Interface Sci 607(Pt 1):423–430
Cong W, Song P, Zhang Y et al (2022) Supramolecular confinement pyrolysis to carbon-supported Mo nanostructures spanning four scales for hydroquinone determination. J Hazard Mater 437:129327
Ma ZP, Zhang L, Ma X et al (2022) A dual strategy for synthesizing crystal plane/defect co-modified BiOCl microsphere and photodegradation mechanism insights. J Colloid Interface Sci 617:73–83
Senasu T, Lorwanishpaisarn N, Hemavibool K et al (2023) Construction of g-C3N4/BiOCl/CdS heterostructure photocatalyst for complete removal of oxytetracycline antibiotic in wastewater. Sep Purif Technol 306:122735
Lin B, Chaturvedi A, Di J et al (2020) Ferroelectric-field accelerated charge transfer in 2D CuInP2S6 heterostructure for enhanced photocatalytic H2 evolution. Nano Energy 76:104972
Sun Y, Cheng H, Gao S et al (2012) Freestanding tin disulfide single-layers realizing efficient visible-light water splitting. Angew Chem Int Ed Engl 51(35):8727–8731
Jiang J, Zhao K, **ao X et al (2012) Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J Am Chem Soc 134(10):4473–4476
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362–381
Guo Y, Zhang R, Zhang S et al (2022) Ultrahigh oxygen-doped carbon quantum dots for highly efficient H2O2 production via two-electron electrochemical oxygen reduction. Energy Environ Sci 15(10):4167–4174
Ding C, Guo J, Chen P et al (2022) All-solid-state Z-scheme In2S3/CQDs/TiO2 heterojunction for highly efficient degradation of ofloxacin. Appl Surf Sci 596:153629
Li H, Liu R, Liu Y et al (2012) Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J Mater Chem 22(34):17470–17475
Shao N, Hou Z, Zhu H et al (2018) Novel 3D core-shell structured CQDs/Ag3PO4@Benzoxazine tetrapods for enhancement of visible-light photocatalytic activity and anti-photocorrosion. Appl Catal B Environ 232:574–586
Li W, Wang Z, Li Y et al (2022) Visible-NIR light-responsive 0D/2D CQDs/Sb2WO6 nanosheets with enhanced photocatalytic degradation performance of RhB: unveiling the dual roles of CQDs and mechanism study. J Hazard Mater 424(Pt C):127595
Hou Y, Laursen AB, Zhang J et al (2013) Layered nanojunctions for hydrogen-evolution catalysis. Angew Chem Int Ed Engl 52(13):3621–3625
Yu C, Li G, Kumar S et al (2014) Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants. Adv Mater 26(6):892–898
Yu C, Yang K, **e Y et al (2013) Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 5(5):2142–2151
Xu M, Yu R, Guo Y et al (2019) New strategy towards the assembly of hierarchical heterostructures of SnO2/ZnO for NO2 detection at a ppb level. Inorg Chem Front 6(10):2801–2809
Di J, **a J, Ge Y et al (2015) Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl Catal B Environ 168–169:51–61
Di J, **a J, Yin S et al (2014) Preparation of sphere-like g-C3N4/BiOI photocatalysts via a reactable ionic liquid for visible-light-driven photocatalytic degradation of pollutants. J Mater Chem A 2(15):5340–5351
Zhu S, Meng Q, Wang L et al (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed Engl 52(14):3953–3957
Duo F, Wang Y, Fan C et al (2016) Enhanced visible light photocatalytic activity and stability of CQDs/BiOBr composites: the upconversion effect of CQDs. J Alloys Compd 685:34–41
**a J, Di J, Li H et al (2016) Ionic liquid-induced strategy for carbon quantum dots/BiOX (X = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl Catal B Environ 181:260–269
Di J, **a J, Ji M et al (2015) The synergistic role of carbon quantum dots for the improved photocatalytic performance of Bi2MoO6. Nanoscale 7(26):11433–11443
Gao X, Wu HB, Zheng L et al (2014) Formation of mesoporous heterostructured BiVO4/Bi2S3 hollow discoids with enhanced photoactivity. Angew Chem Int Ed Engl 53(23):5917–5921
Di J, **a J, Yin S et al (2014) One-pot solvothermal synthesis of Cu-modified BiOCl via a Cu-containing ionic liquid and its visible-light photocatalytic properties. RSC Adv 4(27):14281–14290
Wang S, Li D, Sun C et al (2014) Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl Catal B Environ 144:885–892
Gu X, Yan Q, Wei Y et al (2019) Visible-light-responsive photocatalyst with a microsphere structure: preparation and photocatalytic performance of CQDs@BiOCl. J Mater Sci Mater Electron 30(17):16321–16336
Leelavathi A, Madras G, Ravishankar N (2014) New insights into electronic and geometric effects in the enhanced photoelectrooxidation of ethanol using ZnO nanorod/ultrathin Au nanowire hybrids. J Am Chem Soc 136(41):14445–14455
Zhu Y, Ji X, Pan C et al (2013) A carbon quantum dot decorated RuO2 network: outstanding supercapacitances under ultrafast charge and discharge. Energy Environ Sci 6(12):3665–3675
Di J, **a J, Yin S et al (2013) A g-C3N4/BiOBr visible-light-driven composite: synthesis via a reactable ionic liquid and improved photocatalytic activity. RSC Adv 3(42):19624–19631
Lv Y, Zhu Y, Zhu Y (2013) Enhanced photocatalytic performance for the BiPO4–x nanorod induced by surface oxygen vacancy. J Phys Chem C 117(36):18520–18528
Hu Q, Ji M, Di J et al (2018) Ionic liquid-induced double regulation of carbon quantum dots modified bismuth oxychloride/bismuth oxybromide nanosheets with enhanced visible-light photocatalytic activity. J Colloid Interface Sci 519:263–272
Ye L, Chen J, Tian L et al (2013) BiOI thin film via chemical vapor transport: photocatalytic activity, durability, selectivity and mechanism. Appl Catal B Environ 130–131:1–7
Chen C, Ma W, Zhao J (2010) Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem Soc Rev 39(11):4206–4219
Yu C, Wei L, Zhou W et al (2014) Enhancement of the visible light activity and stability of Ag2CO3 by formation of AgI/Ag2CO3 heterojunction. Appl Surf Sci 319:312–318
Acknowledgements
This work was financially supported by Key Research and Development Project of Anhui Province (No. 2023h11020002), Natural Science Research Project for Universities in Anhui Province (No. KJ2021ZD0006), Natural Science Foundation of Anhui Province (No. 2208085MB21), Fundamental Research Funds for the Central Universities of China (No. PA2022GDSK0056), Anhui Laboratory of Molecule-Based Materials (No. fzj22009), and National Natural Science Foundation of China (Nos. 21725102, 22205108).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there are no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, P., Fang, X., Jiang, W. et al. Coupling of BiOCl Ultrathin Nanosheets with Carbon Quantum Dots for Enhanced Photocatalytic Performance. Trans. Tian** Univ. (2024). https://doi.org/10.1007/s12209-024-00391-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12209-024-00391-4