Abstract
The development of advanced air transportation has raised new demands for high-performance liquid hydrocarbon fuels. However, the measurement of fuel properties is time-consuming, cost-intensive, and limited to the operating conditions. The physicochemical properties of aerospace fuels are directly influenced by chemical composition. Thus, a thorough investigation should be conducted on the inherent relationship between fuel properties and composition for the design and synthesis of high-grade fuels and the prediction of fuel properties in the future. This work summarized the effects of fuel composition and hydrocarbon molecular structure on the fuel physicochemical properties, including density, net heat of combustion (NHOC), low-temperature fluidity (viscosity and freezing point), flash point, and thermal-oxidative stability. Several correlations and predictions of fuel properties from chemical composition were reviewed. Additionally, we correlated the fuel properties with hydrogen/carbon molar ratios (nH/C) and molecular weight (M). The results from the least-square method implicate that the coupling of H/C molar ratio and M is suitable for the estimation of density, NHOC, viscosity and effectiveness for the design, manufacture, and evaluation of aviation hydrocarbon fuels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid hydrocarbon fuels are the dominant energy source in global air transportation, and they have developed rapidly in recent years. However, the sustained and steadily growing demand for air transportation has resulted in the increased aviation fuel consumption, which propels the diversified development of new alternative fuels from non-petroleum resources, e.g., oil sands, oil shale, coal, natural/shale gas, biomass, etc. [1]. Meanwhile, such development also presents new challenges to the performance of liquid hydrocarbon fuels. From a long-term and strategic perspective, the fundamental properties of hydrocarbon fuels should be further evaluated and improved to meet severe requirements. The difference in the physicochemical properties of aviation fuels should be attributed to their varied chemical composition [2]. Thus, the relationship between the chemical composition and properties of hydrocarbon fuels must be understood to support the development of new alternative fuels and enhance the properties of current aviation fuels.
Prior to being used, the physicochemical properties of aviation fuels, such as the density, net heat of combustion (NHOC), low-temperature fluidity, flash point, and thermal-oxidative stability, should be comprehensively evaluated. However, the measurement of fuel properties is time-consuming, cost-intensive, and limited to the operating conditions [3]. By contrast, fuel properties can be predicted based on their chemical composition; prediction of chemical composition requires a small amount of fuel sample. The correlations and predictions of fuel properties based on chemical composition have gained important guidance to the design and synthesis of new high-performance jet fuels.
In general, aviation fuels, such as Jet-A, mainly consist of several hydrocarbons with different molecular structures, including n-paraffins, iso-paraffins, cyclo-paraffins, and aromatics (see detailed composition in Fig. 1), coupled with trace amounts of oxygenates, olefins, and additives [4]. Although the composition–property relationship is complex due to the complex composition of fuels, the fundamental properties of aviation fuels can still be predicted based on their fuel composition [3, 5]. Vozka and Kilaz [3] provided composition analysis approaches, i.e., nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, Raman spectroscopy, and gas chromatography (GC), for the prediction of jet fuel properties.
GC × GC image of Jet-A (reproduced with the permission from Ref .[6])
In this work, we summarized the effects of molecular structure on hydrocarbon properties and the relationship between the chemical composition and properties (including density, NHOC, low-temperature fluidity (viscosity and freezing point), flash point, and thermal-oxidative stability) of aviation fuels (Fig. 2). Additionally, we correlated the fuel properties with hydrogen/carbon molar ratios (nH/C) and molecular weight (M). The results will be helpful for the design, manufacture, and evaluation of new aviation hydrocarbon fuels.
Density
Relationship Between Density and Fuel Composition
Density is a basic aviation fuel property that can influence the loaded fuel weight and aircraft range, which are important for the capacity of aircrafts, especially volume-limited vehicles [7]. Density is also useful in the flow rate calculation and the design of fuel metering system and fuel tank [8]. For aviation turbine fuels, the density is required to be 0.775–0.800 g/cm3 at 15 °C (ASTM D7566-18 [9] and D1655-18 [10]).
Chemical composition determines the density of hydrocarbon fuel. In general, the density of hydrocarbon fuels decreases in the order of aromatics > cyclo-paraffins > paraffins with the same number of carbon atoms, i.e., hydrocarbon fuels with a low H/C molar ratio have a high density. The density of kerosene fractions decreases linearly with the increase in paraffin content (Fig. 3a) [11]. n-Paraffins are generally denser than iso-paraffins because the branching chains can limit the molecular aggregation of hydrocarbons [12].
Fuel density versus a paraffin content of kerosene fractions (reproduced with permission from Ref. [11]), b cyclo-paraffin content of hydrocarbon blends composed of n-, iso-, and cyclo-paraffins (reproduced with permission from Ref. [4]), c total aromatic mass content of RP-3 jet fuels with different hydrogenation degrees (reproduced with permission from Ref. [16]), and d the carbon number of several classes of hydrocarbons (reproduced with permission from Ref. [1])
Fuel density can be promoted by increasing the cyclo-paraffin content. Elmalik et al. [4] observed that the density of the hydrocarbon blend composed of n-, iso-, and cyclo-paraffins showed a linear growth with the increase in cyclo-paraffin content (Fig. 3b). Cyclo-paraffins with cis configuration typically possess a higher density than their trans configurations (symmetry structure) because of the twisted structure of cis configuration, e.g., cis-decalin (0.897 g/cm3) versus trans-decalin (0.870 g/cm3) [13]. For alkyl-substituted cyclo-paraffins, such as methylcyclohexane (0.765 g/cm3), butylcyclohexane (0.796 g/cm3) [14], and l,2-dimethylcyclohexane (0.796 g/cm3) [15], the increased chain length of the alkyl substituent and alkyl substituent number can slightly increase the density. Moreover, the position of alkyl substituents affects the fuel density. Ortho-substituted cyclo-paraffins usually have a higher density compared with meta- and para-substituted cyclo-paraffins due to more compact molecules of the former, e.g., cis-l,2-dimethylcyclohexane (0.796 g/cm3) versus cis-1,4-dimethylcyclohexane (0.783 g/cm3) [15].
Aromatics possess a higher density than paraffins and cyclo-paraffins, and increasing the aromatic content can increase the fuel density [8]. Jia et al. [16] observed that the densities of RP-3 jet fuels with different hydrogenation degrees decreased linearly with the decline in the aromatic content because hydrogenation converted the aromatics into cyclo-paraffins (Fig. 3c). Similar to alkyl-substituted cyclo-paraffins, alkyl substituents in the aromatic ring influence the aromatic density. The decreased distance between alkyl substituents can improve the density of alkyl-substituted aromatic; thus, the density of ortho-substituted aromatic is higher than that of meta- and para-substituted aromatic, e.g., o-xylene (0.875 g/cm3) versus p-xylene (0.857 g/cm3) [17].
Meanwhile, the number of total carbon atoms can also influence the fuel density. As shown in Fig. 3d, the increase in the total carbon number of most hydrocarbons, except alkylbenzene and naphthalene, leads to a high density [1]. The density of alkylbenzene remains almost constant, whereas that of naphthalene declines with the increase in carbon number.
Table 1 compares the density of different hydrocarbon fuels. The relatively high density of RG-1 is possibly due to the high concentration of cyclo-paraffins. Similarly, on account of the high aromatic and cyclo-paraffin contents, JP-8, Jet A, and Jet A-1 possess higher density than JP-4 [18]. Compared with conventional jet fuels, most alternative jet fuels usually have a lower density due to their lower content of aromatics [5]. For instance, the densities of Fischer–Tropsch hydroprocessed synthesized paraffinic kerosene (FT-SPK) and hydroprocessed esters and fatty acids (HEFAs) with less than 0.5wt% of aromatics possess the density of 0.730–0.770 g/cm3, whereas the addition of 20vol% aromatics to FT-SPK can increase the density to 0.800 g/cm3 according to ASTM D7566-18 [9]. Al-Nuaimi et al. [19] used aromatic compounds as additives to improve the GTL SPK density. Although aromatics can increase the fuel density, aviation fuels with a high aromatic content can exhibit decreased ignition tendency, low resistance to extinction [20], and increased soot formation and emissions [21]. Hence, ASTM D7566-18 specifies a maximum aromatic volume content of 25.0% and a minimum content of 8.0% to ensure adequate lubricity [9].
Hydrocarbons with a multi-ring molecular structure possess a high density, which is essential for high-density fuels [32]. Therefore, efforts have been exerted to synthesize high-density fuels that have a multiple-ring structure, such as RJ-4 (0.94 g/cm3), a mixture of exo- and endo-tetrahydrodimethylcyclopentadiene synthesized by hydrogenation of dimethylcyclopentadiene, and JP-10 (0.936 g/cm3) that is an almost pure component of exo-tetrahydrodicyclopentadiene synthesized by hydrogenation of endo-dicyclopentadiene (endo-DCPD) and catalytic isomerization [32,33,34]. Spiro-hydrocarbon fuels with additional rings can exhibit increased density; a high density of 0.952 g/cm3 was reported for spiro[cyclopentane-1,2′-norbornane] (C11H18) synthesized by the zeolite catalytic Mannich–Diels–Alder reaction of biomass and petroleum-derived feedstocks [35], whereas the density of spiro[4,5]decane (C10H18) can reach 0.870 g/cm3 [36]. Notably, liquid diamondoid fuels, such as alkyl adamantanes, alkyl diamantanes, and alkyl triamantanes, also present a high density due to their compact internal molecular structure [37,38,39,40,41].
Strained fuels containing three- or four-membered rings also possess high density. A typical strained fuel is quadricyclane (tetracyclo[3.2.0.02,7.04,6]heptane; QC, 0.983 g/cm3), which contains two three-membered rings and one four-membered ring [42,43,44,45]. Moreover, cyclopropanation, which was first reported by Simmons and Smith [46, 47] through the reaction of alkenes with diiodomethane and a zinc-copper couple, is an effective method for the formation of three-membered carbocyclic rings to increase the fuel density. Oh et al. [48] reported that the density of a series of cyclopropane-fused hydrocarbons synthesized by cyclopropanation of norbornene and DCPDs can reach 0.940–1.040 g/cm3. Therefore, the construction of a multi-ring structure is an important approach to obtain high-density fuels.
Based on the above demonstrations, fuel density is closely related to the molecular structure, total carbon number, and H/C molar ratio of fuels.
Correlation and Estimation of Density
On the account of a hydrocarbon fuel mixture, Eq. (1) gives an effective method to predict the density of a hydrocarbon mixture, showing a linear function of the mass fraction of each component [49].
where ρmix is the mixture density, and xi and ρi are the mass fraction and density of ith component, respectively.
Correlation of Density and Composition of Fuels
Fuel density is generally dependent on the chemical composition. The fuel composition should be first analyzed by several approaches before the correlations of fuel density and the detailed composition are established.
Cookson et al. [50, 51] determined the mass fractions of n-paraffins, cyclo-paraffins combined with iso-paraffins, and aromatics through 13C NMR, high-performance liquid chromatography (HPLC), and GC to establish the linear expressions of composition and fuel density by multi-linear regression (MLR) analysis (Entries 1–4, Table 2). Then, the aromatic carbon and n-alkyl carbon contents were introduced to predict the fuel density (Entries 5–10, Table 2) [52, 53]. Liu et al. [54] determined the detailed composition of 80 jet fuels by GC–mass spectrometry (GC–MS) and categorized the chemical composition into eight hydrocarbon classes. With the help of an artificial neural network (ANN), they presented the multiple linear correlations of composition–property of fuels to predict fuel density (Entries 11–12, Table 2). Furthermore, Shi et al. [55] used two-dimensional GC with MS and flame ionization detector (GC × GC–MS/FID) to accurately measure the aviation fuel composition, which was sorted into 10 hydrocarbon classes and carbon numbers (C7–C19); they further developed the quantitative composition–property relationship of aviation fuels by different statistical algorithms, including weighted average (WA) method, partial least squares (PLS) analysis, genetic algorithm, and modified WA method. Moreover, the PLS regression models based on near-IR or Fourier transform IR spectroscopy (FT-IR) spectra can provide a practical method to predict fuel density [56,57,58]. Al-Nuaimi et al. [19] predicted the density of hydrocarbon mixture with mass fractions of n-, iso-, and cyclo-paraffins (Entry 13, Table 2). Gülüm et al. [59] proposed one- and two-dimensional regression models to predict the density of fuel blends based on the fuel composition and temperature. To simplify the correlations of composition–property, Yue et al. [60] proposed a unique regression model that correlates the density with H/C molar ratios and M of hydrocarbon fuels (Entry 14, Table 2).
Correlation of Density Versus H/C Molar Ratio and M
The saturation degree of hydrocarbon molecular can be reflected by the H/C molar ratio. A high H/C molar ratio of hydrocarbon implies the decrease in molecular ring structures, especially aromatic ring structures, in aviation fuel [61], which will decrease the fuel density. Moreover, the M is a significant parameter for describing the molecular structure and the total carbon number of hydrocarbons. To simplify the correlation for practical application, we correlated the density data of 64 kinds of fuels, including aviation, synthetic hydrocarbon, alternative, and bio-jet fuels, with their H/C molar ratios and M (Table S1, Supporting Information, SI) by the least-square method.
Figure 4 shows the relationship between the density and nH/C /M0.19 of 64 hydrocarbon fuels, and Eq. (2) gives the linear regression equation for these fuels with a deviation of 6%. The result shows that the individual hydrocarbon fuels strictly follow the same tendency, i.e., the density decreases with the increase in nH/C /M0.19, and hydrocarbon fuels with the lower H/C molar ratio and higher M are prone to possess higher density.
where ρ is the density of hydrocarbon fuels in g/cm3, and nH/C and M are the hydrogen-to-carbon molar ratio and average M of selected hydrocarbon fuels, respectively.
NHOC
NHOC is the released energy obtained from the complete combustion of fuel, with products existing in the gaseous state [62]. As an indicator of the energy content of aviation fuels, NHOC directly determines the flying distance and payload of flight and is important for the combustion characteristics, stability, efficiency, and emissions of aviation fuels [63]. The NHOC can be represented by the gravimetric and volumetric NHOC. For most civil aircrafts with a restricted payload weight, the effective payload must be promoted by increasing the gravimetric NHOC. On the contrary, for volume-limited aircraft vehicles, such as rockets and missiles, in which the fuel tank must be designed to be small enough to save sufficient space for the electronic components and electrical equipment, the priority is to increase the volumetric NHOC for maximum flight range [32, 64, 65].
Gravimetric NHOC
Relationship Between Gravimetric NHOC and Fuel Composition
The gravimetric NHOC should be higher than 42.8 MJ/kg for conventional fuels and/or fuel blends to meet ASTM D7566-18 or D1655-18. However, no such restriction exists for synthetic fuels, except the synthesized iso-paraffin fuels with a minimum requirement of 43.5 MJ/kg.
The gravimetric NHOC is positively related to the hydrogen content (H/C molar ratio) of fuels [66,67,68]; more energy can be released from the complete combustion of hydrogen atoms per unit mass compared with carbon atoms. In general, the gravimetric NHOC of different types of hydrocarbons decreases in the following order: paraffins > cyclo-paraffins > aromatics with the same carbon number (Fig. 5) [1]. The molecular structure of n- and iso-paraffins and the position of alkyl substituents (e.g., 2- and 3-methylalkanes) have a minor effect on the gravimetric NHOC of paraffins due to the same H/C molar ratio. Moreover, the gravimetric NHOC of n-paraffins and iso-paraffins decreases with the increase in the carbon number owing to the reduced H/C molar ratio. However, for cyclo-paraffins and alkylbenzenes, the increase in the total carbon number leads to an almost constant and high gravimetric NHOC, respectively [1], because the H/C molar ratio of cyclo-paraffins remains constant, whereas that of the aromatics increases with the rise in carbon atom number.
Gravimetric NHOC versus the total carbon number of hydrocarbons (reproduced with permission from Ref. [1])
Conventional jet fuels with high concentration of aromatics and cyclo-paraffins normally exhibit a low gravimetric NHOC [2] because of the low H/C ratio caused by the cyclic structure and unsaturated bonds [19]. As shown in Table 1, S-8 with 99.0 wt% saturated paraffins exhibits a higher gravimetric NHOC (44.1 MJ/kg) than other hydrocarbon fuels containing aromatics. Jia et al. [16] reported that the hydrogenation of Chinese RP-3 jet fuel can increase the gravimetric NHOC, which is negatively related to the aromatic content in linearity, from 43.0 MJ/kg to 43.2 MJ/kg. In addition, bio-jet fuels possess a higher gravimetric NHOC than conventional jet fuels due to the high concentrations of n- and iso-paraffins.
The high density of hydrocarbon fuels can be achieved at the cost of reducing the hydrogen content, leading to a low gravimetric NHOC [32]. For example, perhydrofluorene, which contains one five-membered ring and two six-membered rings, has a higher density (0.959 g/cm3) [69] than decalin (0.881 g/cm3) [60]; however, its gravimetric NHOC (41.81 MJ/kg) is lower than that of decalin (42.59 MJ/kg). Differently, strained hydrocarbon fuels (Table 3) can attain high density and gravimetric NHOC due to the extra strain energy stored in their three- or four-membered rings. The gravimetric NHOC of tricyclo[3.2.1.02,4]octane (0.94 g/cm3, 41.19 MJ/kg) with a three-membered ring is lower than that of tetracyclo[3.3.1.02,4.06,8]nonane (1.00 g/cm3, 43.09 MJ/kg) with two three-membered rings [48], indicating that increasing the number of strained rings leads to increased density and gravimetric NHOC. From Table 3, strained fuels with a strained ring molecular structure and low H/C molar ratio exhibit a high gravimetric NHOC, which is contrast to the result for multi-cyclic hydrocarbon fuel.
Correlation and Estimation of Gravimetric NHOC
The gravimetric NHOC of blended hydrocarbon fuels can be calculated from the NHOC of individual hydrocarbon components using Eq. (3).
where NHOCi and xi are the NHOC in MJ/kg and mass fraction of ith fuel component, respectively.
ASTM D3338/D3338M-20 [71] and ASTM D4529-17 [72] provide the predicted gravimetric NHOC of hydrocarbon fuels (Entries 1–2, Table 4). Al-Nuaimi et al. [19] calculated the gravimetric NHOC of hydrocarbon mixtures with the mole fractions of n-, iso-, and cyclo-paraffins (Entry 3, Table 4). In view of the strong relationship between NHOC and hydrogen content, Antoine et al. [67] correlated the NHOC with the hydrogen content of different synthetic fuels (Entry 4, Table 4). Analogously, the H/C molar ratio was also used to predict the NHOC of different hydrocarbon fuels (Entry 5, Table 4) [60]. Similar to the prediction of fuel density, HPLC, 13C NMR, FT-IR spectra, GC, GC–MS, and GC × GC–MS/FID have been conducted to determine the fuel composition. Then, the correlations of gravimetric NHOC with the detailed composition can be established by different regression methods, such as the linear regression method, ANN approaches, and statistical algorithms. Cookson et al. [50,51,52] used HPLC, GC, and 13C NMR to determine the fuel composition and correlated the gravimetric NHOC with the detailed composition by the linear regression method (Entries 6–10, Table 4). Liu et al. [54] measured the fuel composition by GC–MS and further developed the relationship of composition–gravimetric NHOC by the ANN approach (Entries 11–12, Table 4). Shi et al. [55] determined the aviation fuel composition through GC × GC–MS/FID and correlated the gravimetric NHOC with the detailed composition by different statistical algorithms. In addition, PLS regression models combined with FT-IR spectra were used for the prediction of the gravimetric NHOC of hydrocarbon fuels [73].
As discussed, the gravimetric NHOC of aviation hydrocarbon fuels is closely related to their hydrogen content. Thus, the H/C molar ratio is an important parameter for the correlation of gravimetric NHOC. Meanwhile, the number of total carbon atoms also affect fuel gravimetric NHOC. Thus, we correlated the gravimetric NHOC with H/C molar ratios and M of 64 hydrocarbon fuels (Table S1, SI) by the least-square method. As shown in Fig. 6, the correlation can be divided into two different groups: multi-cyclic hydrocarbon fuels containing five- or six-membered rings (red line) and strained hydrocarbon fuels with three- or four-membered rings (black line). For the former group, the relationship between gravimetric NHOC and nH/C /M0.02 for multi-cyclic hydrocarbon fuels containing five- or six-membered rings can be expressed as Eq. (4).
where NHOC is the net heat of combustion per unit mass for hydrocarbon fuel in MJ/kg and nH/C is the hydrogen to carbon molar ratio.
From Eq. (4), a positively linear correlation exists between the gravimetric NHOC of most multi-cyclic hydrocarbon fuels and nH/C /M0.02 with an error of 2.5%, which proves that the H/C molar ratio determines the gravimetric NHOC for multi-cyclic hydrocarbons.
However, for the strained hydrocarbon fuels with three- or four-membered rings (Table 3), the correlation between gravimetric NHOC and nH/C /M0.02 exhibits a negatively linear correlation, as described in Eq. (5).
Although the increase in three- or four-membered rings reduces the H/C molar ratio, the stored strain energy contributes considerable combustion energy to the gravimetric NHOC. Thus, the gravimetric NHOC of strained fuel is negatively related to its H/C molar ratio.
Volumetric NHOC
Correlation Between Volumetric NHOC and Density
We correlated the volumetric NHOC of 59 hydrocarbon fuels (Table S1, SI) with the fuel density by using the least-square method, and the results are shown in Fig. 7. The volumetric NHOC of hydrocarbon fuels increases linearly with density (with R2 of 0.98, Eq. (6)).
where NHOC and ρ are the volumetric NHOC in MJ/L and density in g/cm3, respectively.
Given the positively linear correlation between the volumetric NHOC and fuel density, many studies attempted to synthesize high-density fuels to increase the volumetric NHOC and expand the flight distance [32, 35, 36, 69, 74, 75]. A crucial step toward high-density fuels is the synthesis of RJ-4 (39.0 MJ/L) with a density of 0.940 g/cm3 [34]. As the most widely used missile fuel, JP-10 (0.936 g/cm3) has a NHOC of 39.40 MJ/L [33], whereas QC (0.983 g/cm3), as a strained hydrocarbon fuel, has a higher NHOC (43.58 MJ/L) [45]. RJ-5 (the isomer of dihydrodinorbornadiene, 1.080 g/cm3) is the first aviation hydrocarbon fuel with a NHOC of 44.90 MJ/L; it is synthesized from cycloaddition reactions of norbornadiene, followed by hydrogenation and isomerization [34].
Correlation of Volumetric NHOC Versus H/C Molar Ratio and M
Given that the H/C molar ratio and M can serve as parameters to determine the fuel density, we correlated the volumetric NHOC with H/C molar ratios and M based on 59 hydrocarbon fuels (Table S1, SI); Eq. (7) describes the negative linear equation between the volumetric NHOC of hydrocarbons and nH/C /M0.19. The result in Fig. 8 shows that the volumetric NHOC decreases linearly with nH/C /M0.19 with R2 of 0.93. Comparing Figs. 4 and 8, the density and volumetric NHOC present an excellent linear relationship with nH/C /M0.19, confirming the positive relationship between the volumetric NHOC and density, consistent with the result in Fig. 7.
Low-Temperature Fluidity
Low-temperature fluidity, which can be quantitatively reflected and described by the low-temperature viscosity and freezing point of aviation fuels, is a vital character for the application of fuels, especially in environments with low temperature and high altitudes.
Viscosity
Relationship Between Viscosity and Fuel Composition
Viscosity is a fundamental physical property to quantitatively evaluate the fluidity of aviation fuel [65, 76,77,78]. ASTM D7566-18 specifies that the kinematic viscosity should not exceed 8.00 mm2/s (cst) at − 20 °C for aviation fuels, such as Jet A and Jet A-1. In general, the viscosity of hydrocarbon fuels increases with the M (total carbon number) [76]. As shown in Table 1, the viscosity of JP-5 is 6 mm2/s at − 20 °C, which is higher than those of JP-8 (4.10 mm2/s) and Jet A (5.20 mm2/s), due to its higher average M (166.3 g/mol). For aliphatic hydrocarbon fuels, the kinematic viscosity increases with the carbon chain length [77].
Cai et al. [79] proposed a quantitative structure–property relationship (QSPR) model to estimate and compare the viscosity (predicted and experimental) of different hydrocarbon class homologs (Fig. 9). The viscosity of n-paraffins, iso-paraffins, cyclo-paraffins, olefins, and aromatic homologs increases with the number of carbon atoms at a given temperature. With the same carbon number, cyclo-paraffins and aromatics are more viscous than paraffins [15, 61, 76]. Cyclo-paraffins with cis configuration have a higher viscosity than their trans configuration, e.g., cis-decalin (2.52 mm2/s) versus trans-decalin (1.75 mm2/s) at 40 °C [77], probably due to the increased molecular interaction caused by the twisted structure of cis configuration. The alkyl substituents also affect the viscosity of cyclo-paraffins. The increase in chain length of alkyl substituents increases the viscosity of alkyl-substituted cyclo-paraffins, e.g., methylcyclohexane (0.88 mm2/s) versus butyl-cyclohexane (1.22 mm2/s) at 40 °C [77]. Similar to density, viscosity can be affected by the position of alkyl substituents. Ortho-substituted cyclo-paraffins generally exhibit higher viscosity than meta- and para-substituted cyclo-paraffins possibly because of the more compact molecular structure of ortho-substituted cyclo-paraffins, e.g., cis-l,2-dimethylcyclohexane (1.40 mm2/s) versus cis-l,4-dimethylcyclohexane (1.12 mm2/s) at 20 °C [15]. n-Paraffins generally have a slightly higher viscosity than iso-paraffins. Geist and Cannon [15] evaluated the viscosity of 18 chemical isomers of octane and observed that not all isomeric octanes exhibit lower kinematic viscosity than n-octane, indicating that the branching level of saturated hydrocarbons can also affect fuel viscosity.
Viscosity (experimental and predicted by the proposed QSPR model) of n-paraffin, iso-paraffin, cyclo-paraffin, olefin and aromatic homologs versus temperature (reproduced with permission from Ref. [79])
Aromatics can increase the aviation fuel viscosity [80], and their contributions to fuel viscosity vary with their molecular size and structures. Solash et al. [68] observed that the kinematic viscosity of coal-derived fuels increases steadily in a linear manner with the average molecular size of aromatics. Dixonand Clark [81] reported that the viscosity of alkylbenzenes increases with the number of substituted methyl groups, because the hyperconjugation effect of the aromatic ring and methyl group restricts the flow of alkylbenzenes. The position of alkyl substituents can also affect the aromatic viscosity. Similar to cyclo-paraffins, ortho-substituted aromatics usually have a higher viscosity compared with meta- and para-substituted aromatics, e.g., o-xylene (0.922 mm2/s) versus p-xylene (0.784 mm2/s) at 20 °C [15]. Overall, fuel viscosity is determined by the total carbon number, hydrocarbon class, and molecular structure.
Correlation and Estimation of Viscosity
As a thermodynamic property, the viscosity of blended fuels can be estimated by the Grunberg-Nissan equation [82,83,84]:
where ηmix and ηi are the dynamic viscosity of fuel mixture and ith fuel component in mPa·s, respectively. xi and xj are the molar fractions of the fuel components i and j, respectively. Gij is the interaction parameter between ith and jth fuel component in mPa s, and n is the total number of fuel components in the mixture.
Many studies attempted to estimate viscosity on the basis of molecular structure [56, 57, 73, 79]. Cai et al. [79] proposed a QSPR model to predict the hydrocarbon viscosity based on the basic groups, united group, and M of fuels. To simplify the relationship of viscosity and fuel composition, Yue et al. [60] correlated viscosity with the H/C molar ratios and M of hydrocarbon fuels by using an exponential regression model. To broaden the applicability of the model, we correlated the kinematic viscosities of 23 hydrocarbon fuels at − 20 °C (Table S1, SI) with H/C molar ratios and M by the exponential regression method (Fig. 10). The exponential regression (Eq. (9)) for kinematic viscosity and nH/C /M 0.54 indicates that the hydrocarbon fuels with low H/C molar ratios and high M preferentially have high viscosities.
where v is the kinematic viscosity of hydrocarbon fuel in mm2/s.
Freezing Point
Relationship Between Freezing Point and Fuel Composition
Freezing point, as a key property to describe the low-temperature fluidity of fuel, is the temperature at which the solid wax and ice crystals formed in the previous cooling vanish when heated slowly [50, 85]. Aviation fuels operated at extremely low temperatures (or high altitudes) require a low freezing point to avoid the formation of hydrocarbon crystals that can cause clogging of the fuel supply system and other machine or operation problems [86, 87]. The freezing point of Jet A should be lower than − 40.0 °C, whereas the requirement for Jet A-1 is specified to be lower than − 47.0 °C according to ASTM D7566-18. The freezing point of hydrocarbon fuel is a crystallization behavior, in which molecular symmetry and intermolecular forces play the key roles [55, 88]. By measuring the freezing point of hydrocarbon mixtures, Affens et al. [86] reported that the data derived from Van’t Hoff equation of certain hydrocarbon mixtures were not in agreement with the literature data due to the strong intermolecular forces of the mixtures.
One important factor affecting the freezing point is the carbon chain length of hydrocarbon fuels; the freezing point of hydrocarbons increases with the number of total carbon atoms [88, 89]. Thus, catalytic hydrocracking of aviation fuels is a promising method to reduce the freezing point by shortening the carbon chain length [94])
The effect of aromatic components on the freezing point has been also investigated. Hong et al. [94] noted that the addition of propylbenzene can reduce the freezing point of bio-jet fuel mixture (Fig. 11c). Similarly, Al-Nuaimi et al. [19] observed that GTL SPK blended with the conventional jet fuels has a low freezing point owing to the introduction of aromatics. The position of alkyl substituents can also affect the freezing point of aromatics. For instance, p-xylene (13.2 °C) exhibits a notably higher freezing point than o-xylene (− 25.2 °C) and m-xylene (− 47.9 °C) due to its better molecular symmetry.
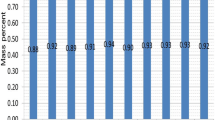
In addition to the molecular structure, the average carbon number influences the freezing point. As shown in Table 1, JP-4 has the lowest freezing point temperature (− 62.2 °C) among the summarized fuels due to its relatively low average number of carbon atoms. S-8 and Chinese RP-3 jet fuels have a high average carbon number, but their freezing points are as low as − 59.0 °C and − 58.0 °C, respectively, owing to the high concentration of iso-paraffins, i.e. 82.0 wt% for S-8 [22] and 42.7 wt% for RP-3 [23].
Overall, hydrocarbon fuels with high molecular symmetry and intermolecular force generally have a high freezing point. Specifically, the freezing point of hydrocarbon increases with the number of total carbon atoms. For the same average carbon number, iso-paraffins and aromatics possess relatively low freezing points, whereas n-paraffin fuels show a high freezing point. Hence, the catalytic hydrocracking and hydroisomerization of aviation fuels are major methods used to reduce the freezing point.
Correlation and Estimation of Freezing Point
Al Mulla and Albahri [95] proposed an empirical method to calculate the freezing point based on the blending freezing point index (Ifr,blend), which is determined by the freezing point index of each fuel component, that can be calculated by the following equation:
where Ii and Tfr,i are the freezing point index and the freezing point of ith fuel component in K, respectively. The blending freezing point index (Ifr,blend) can be obtained by Eq. (11).
where vi is the volume fraction of the ith fuel component, and Ifr,blend is the freezing point index of the fuel blend. Therefore, the freezing point of fuel blends can be calculated by Eq. (12).
where Tfr is the freezing point of the fuel blend in K.
Cookson et al. [50, 52, 53] used the MLR method to correlate the freezing point and the fuel composition detected by HPLC, GC, and 13C NMR (Entries 1–4, Table 5); the boiling range was introduced to develop several expressions (Entry 5, Table 5) [53]. Liu et al. [54] used GC–MS to determine the content of eight hydrocarbon classes and further correlated the freezing point by the ANN method (Entries 6–7, Table 5). Moreover, Shi et al. [55] classified the aviation fuel composition under 10 hydrocarbon classes and using the carbon number (C7–C19) obtained by GC × GC–MS/FID and used different statistical algorithms to correlate the freezing point and chemical composition. Hong et al. [94] correlated the freezing point of fuel blends with the volume content of propylbenzene (Entry 8, Table 5). Al-Nuaimi et al. [19] proposed several multiple nonlinear correlations between the freezing point and the composition of fuel blends composed of n-, iso- and cyclo-paraffins to predict the freezing points of blending fuels (Entry 9, Table 5). Analogous to the density and NHOC, PLS regression method based on FT-IR spectra can also predict the fuel freezing point [58].
To simplify the relationship between the freezing point and molecular structure of aviation fuels, we attempted to correlate the freezing point with H/C molar ratios and M; however, we failed to obtain a suitable model probably given the difficulty of reflecting molecular symmetry and intermolecular forces [55, 88].