Abstract
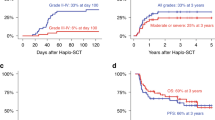
An optimal pretransplant conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT) in older adults has not been established. Three prospective multicenter phase II studies were conducted, in which 142 patients older than 54 years (median age, 61 years; range 55–70 years) with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) received a myeloablative dose of intravenous busulfan (ivBu, 12.8 mg/kg) along with fludarabine (180 mg/m2) ± low dose total body irradiation for allo-HSCT between September 2009 and February 2013. A total of 103 AML and 39 MDS patients including 21 related bone marrow (BM) or peripheral blood (PB), 50 unrelated BM, and 71 unrelated cord blood (UCB) transplantation were enrolled. Grade 3 or greater toxicities were observed in 105 patients. Neutrophil engraftment was achieved in 70 out of the 71 related PB/BM or unrelated BM recipients, and 61 out of the 71 UCB recipients. The cumulative incidence rates of relapse and non-relapse mortality after 2 years were 24.0 and 24.1%, respectively. The overall and event-free survival rates at 2 years were 53.3 and 47.4%, respectively. The myeloablative dose of ivBu was well tolerated without increased toxicity-related mortality in older adults who underwent allo-HSCT with any donor source.




Similar content being viewed by others
References
Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–7.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–400.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–63.
Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–11.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304–12.
Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2016;22(4):651–7.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154–61.
Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(5):523–36.
Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102(3):820–6.
Yeh RF, Pawlikowski MA, Blough DK, McDonald GB, O'Donnell PV, Rezvani A, et al. Accurate targeting of daily intravenous busulfan with 8-hour blood sampling in hospitalized adult hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2012;18(2):265–72.
Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104(5):1574–7.
Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Theoret Y, Champagne MA, et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant. 2010;45(2):261–7.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–64.
Andersson BS, de Lima M, Saliba RM, Shpall EJ, Popat U, Jones R, et al. Pharmacokinetic dose guidance of iv busulfan with fludarabine with allogeneic stem cell transplantation improves progression free survival in patients with AML and MDS; results of a randomized phase III study. In: 53rd ASH annual meeting and exposition, Orlando, FL, 2011.
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM, et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005;35(1):17–23.
Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(9):993–1003.
Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6):672–84.
Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, **ao L, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17(10):1490–6.
Chantepie SP, Gac AC, Reman O. Feasibility of the fludarabine busulfan 3 days and ATG 2 days reduced toxicity conditioning in 51 allogeneic hematopoietic stem cell transplantation: a single-center experience. Leuk Res. 2014;38(5):569–74.
Oudin C, Chevallier P, Furst S, Guillaume T, El Cheikh J, Delaunay J, et al. Reduced-toxicity conditioning prior to allogeneic stem cell transplantation improves outcome in patients with myeloid malignancies. Haematologica. 2014;99(11):1762–8.
Kawamura K, Kako S, Mizuta S, Ishiyama K, Aoki J, Yano S, et al. Comparison of conditioning with fludarabine/busulfan and fludarabine/melphalan in allogeneic transplantation recipients 50 years or older. Biol Blood Marrow Transplant. 2017;23(12):2079–87.
Magenau J, Tobai H, Pawarode A, Braun T, Peres E, Reddy P, et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood. 2011;118(15):4258–64.
Magenau J, Westervelt P, Khaled S, McGuirk J, Hari P, Eapen M, et al. A multicenter trial of myeloablative clofarabine and busulfan conditioning for relapsed or primary induction failure AML not in remission at the time of allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(1):59–655.
Horwitz ME, Morris A, Gasparetto C, Sullivan K, Long G, Chute J, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant. 2008;14(5):591–4.
Komatsu T, Narimatsu H, Yoshimi A, Kurita N, Kusakabe M, Hori A, et al. Successful engraftment of mismatched unrelated cord blood transplantation following reduced intensity preparative regimen using fludarabine and busulfan. Ann Hematol. 2007;86(1):49–544.
Yamamoto H, Uchida N, Matsuno N, Kon A, Nishida A, Ota H, et al. I.v. BU/fludarabine plus melphalan or TBI in unrelated cord blood transplantation for high-risk hematological diseases. Bone Marrow Transplant. 2015;50(4):607–9.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–9.
Yamamoto H, Uchida N, Yuasa M, Kageyama K, Ota H, Kaji D, et al. A novel reduced-toxicity myeloablative conditioning regimen using full-dose busulfan, fludarabine, and melphalan for single cord blood transplantation provides durable engraftment and remission in nonremission myeloid malignancies. Biol Blood Marrow Transplant. 2016;22(10):1844–50.
Uchida N, Hidaka M, Sakura T, Miyamoto T, Fujisaki T, Eto T, et al. Prospective multicenter phase II study of myeloablative conditioning consisting of intravenous busulfan and fludarabine +/− total body irradiation for older patients (55 years and older): final analysis of the JSCT FB09 study. In: BMT Tandem meetings 2014, Grapevine, TX, 2014.
McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res. 2014;20(3):754–63.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83.
Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours. 3rd ed. Lyon: IARC Press; 2001.
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706.
Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28(10):909–15.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;116:1141–54.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34(2):187–220.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103(1):11–9.
Chow DS, Bhagwatwar HP, Phadungpojna S, Andersson BS. Stability-indicating high-performance liquid chromatographic assay of busulfan in aqueous and plasma samples. J Chromatogr B Biomed Sci Appl. 1997;704(1–2):277–88.
Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37(4):345–51.
Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439–46.
Flowers CR, Costa LJ, Pasquini MC, Le-Rademacher J, Lill M, Shore TB, et al. Efficacy of pharmacokinetics-directed busulfan, cyclophosphamide, and etoposide conditioning and autologous stem cell transplantation for lymphoma: comparison of a multicenter phase II study and CIBMTR outcomes. Biol Blood Marrow Transplant. 2016;22(7):1197–205.
Buxton ILO, Benet LZ. Pharmaokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill; 2010. p. 17–39.
Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168(4):481–91.
Peters WP, Henner WD, Grochow LB, Olsen G, Edwards S, Stanbuck H, et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res. 1987;47(23):6402–6.
Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant. 2012;47(1):5–14.
Mehta RS, Di Stasi A, Andersson BS, Nieto Y, Jones R, de Lima M, et al. The development of a myeloablative, reduced-toxicity, conditioning regimen for cord blood transplantation. Clin Lymphoma Myeloma Leuk. 2014;14(1):e1–5.
Sanz J, Picardi A, Hernandez Boluda JC, Martin C, Ferra C, Nozzoli C, et al. Impact of graft-versus-host disease prophylaxis on outcomes after myeloablative single-unit umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(9):1387–92.
El Kourashy S, Williamson T, Chaudhry MA, Savoie ML, Turner AR, Larratt L, et al. Influence of comorbidities on transplant outcomes in patients aged 50 years or more after myeloablative conditioning incorporating fludarabine. BU and ATG Bone Marrow Transplant. 2011;46(8):1077–83.
Daly A, Savoie ML, Geddes M, Chaudhry A, Stewart D, Duggan P, et al. Fludarabine, busulfan, antithymocyte globulin, and total body irradiation for pretransplantation conditioning in acute lymphoblastic leukemia: excellent outcomes in all but older patients with comorbidities. Biol Blood Marrow Transplant. 2012;18(12):1921–6.
Oh H, Loberiza FR Jr, Zhang MJ, Ringden O, Akiyama H, Asai T, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105(4):1408–16.
Chen YB, Coughlin E, Kennedy KF, Alyea EP, Armand P, Attar EC, et al. Busulfan dose intensity and outcomes in reduced-intensity allogeneic peripheral blood stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19(6):981–7.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9.
Versluis J, Labopin M, Niederwieser D, Socie G, Schlenk RF, Milpied N, et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia. 2015;29(1):51–7.
Acknowledgements
The authors thank all physicians, nurses, pharmacists, and support personnel in the participating institutes for their care of patients in this study. The authors also thank the staff members of the JSCT office for outstanding data management. This work was supported by Grants from the Research Foundation for Community Medicine. The ivBu PK study was supported by Grants from the Japanese Ministry of Health, Labour and Welfare (H22-018).
Author information
Authors and Affiliations
Consortia
Contributions
NU, TM, TT, ST, TY, S-IM, and MH designed the clinical protocol; KM and KM designed the PK protocol and did measurement and analysis of serum concentration of ivBu; JK conducted the statistical analysis; NU, TS, MH, TM, TE, YM, TM, NF, and GY enrolled and cared for the patients; NU and MH supervised the overall conduction of the study; NU and KM wrote the manuscript; KA and MH critically reviewed the manuscript and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Uchida, N., Matsumoto, K., Sakura, T. et al. Myeloablative intravenous busulfan-containing regimens for allo-HSCT in AML or MDS patients over 54 years old: combined results of three phase II studies. Int J Hematol 112, 510–523 (2020). https://doi.org/10.1007/s12185-020-02941-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02941-7